DriveAFM — Performance without compromise
- Overview
- WaveMode
- Life science
- Materials science
- SMM
- FluidFM
- PicoBalance
- Glovebox
- Accessories
- Software
- Related content
Overview
DriveAFM — Performance without compromise
The DriveAFM is Nanosurf’s novel flagship AFM platform: a tip-scanning atomic force microscope (AFM) that combines, for the first time, several capabilities in one instrument to enable novel measurements in materials and life sciences. The DriveAFM overcomes drawbacks of other tip-scanning instruments and provides atomic resolution together with fast scanning, fast force spectroscopy, and large scan sizes up to 100 µm. Thanks to Nanosurf’s innovations in optical beam path engineering and scanner design, the DriveAFM scan head features photothermal actuation and full motorization for superior research performance and is easy to use for researchers at all levels of experience.
CleanDrive: stable excitation in air and liquid
Ultra-low noise
Direct drive: high-resolution imaging and large scan area
Fully motorized system: full control via software

Download the DriveAFM Brochure
Get a printable PDF of our flagship AFM's brochure and dive deep into the technology, examples of application and measurements on different samples, and system specifications.

CleanDrive: stability in air and liquid with photothermal excitation
Photothermal excitation of the cantilever provides unparalleled stability, a linear frequency response, and a high excitation bandwidth in air and liquid environments. These benefits allow measurements at multiple frequencies and highspeed applications and open new horizons for innovative new measurement modes (e.g. PicoBalance).
These advantages are amplified in liquids since only the cantilever beam is excited and the liquid environment remains largely unperturbed. This results in clean resonance peaks and not the “forest of peaks” commonly seen with the piezo acoustic excitation of cantilevers. Furthermore, this method of exciting the cantilever is insensitive to changes in the environment and distance to the sample, making the whole measurement system much more stable.

The DriveAFM, with its small light spots, is compatible with the use of small cantilevers, which have several advantages that make them superior in performance. While they have the same spring constant as a conventional cantilever, small cantilevers show a significantly higher resonance frequency and operational bandwidth. Also, the noise performance is better. Due to the small dimensions, the sensitivity is increased, and hydrodynamic drag is decreased. All of this results in better imaging performance.
Ultra-low noise
The DriveAFM has a very low overall noise floor, which is achieved through a combination of a low-noise/low-coherence superluminescent diode and a low-noise/high-bandwidth photodetector used in the beam deflection detection module and the low-noise/high-bandwidth CX Controller. This is the basis for the stable, sensitive, and high-resolution imaging and force spectroscopy capability of the DriveAFM.
Direct drive scanner
The DriveAFM exploits the power of direct drive piezo actuation. The 1:1, non-geared actuation scheme of the DriveAFM’s flexure scanner provides more force and can drive stiffer scanners. The resulting higher resonance frequency of the scanner components allows for a higher available actuation bandwidth than with geared drives of the same scan size. The direct drive scanner actuation in combination with the low noise 28-bit CX Controller allows for both imaging at large scales and at high resolution. The DriveAFM is the perfect solution for high-resolution imaging of demanding samples such as nanostructures, proteins, or polymeric structures (e.g. DNA), and also for larger, micrometer-sized structures.
Full motorization
The DriveAFM is the first fully motorized AFM system that can be integrated with an inverted optical microscope. The adjustment of the two light sources for the beam deflection detection system and the CleanDrive photothermal excitation, as well as the photodetector, are fully motorized and can be controlled from the software. The tip approach to the sample is also motorized. The full motorization not only contributes to the ease of use but also allows new possibilities to fully automate the system.
DriveAFM imaging modes
This overview shows which modes the instrument is capable of. Some modes may require additional components or software options. For details, please view the brochure or contact us directly.
Standard imaging modes
Static Force Mode
Lateral Force Mode
Dynamic Force Mode (Tapping Mode)
Phase Imaging Mode
Magnetic properties
Magnetic Force Microscopy
Electrical properties
Conductive AFM (C-AFM)
Piezoelectric Force Microscopy (PFM)
Electrostatic Force Microscopy (EFM)
Kelvin Probe Force Microscopy (KPFM)
Scanning Microwave Microscopy (SMM)
Mechanical properties
Force Spectroscopy
Force Modulation
Stiffness and Modulus
Adhesion
Unfolding and Stretching
Force Mapping
Lithography and Nanomanipulation
Electrochemical AFM (EC-AFM)
WaveMode
WaveMode: Off-resonance imaging remastered
WaveMode is Nanosurf’s new imaging mode, developed in a collaboration with the research groups of Georg Fantner (EPFL) and Matthias Rosenthal (ZHAW). It provides additional ease of use for users from all backgrounds. It is the first commercially available off-resonance imaging mode using CleanDrive photothermal excitation that is itself well-known for its exceptional excitation stability and bandwidth in any environment. Founded on these advantages, WaveMode allows stable and fast operation in all environments.
WaveMode: Off-resonance imaging remastered
WaveMode is Nanosurf’s new off-resonance imaging mode using the power of CleanDrive photothermal excitation. The first implementation of photothermal off-resonance tapping was set up by Georg Fantner at the EPFL. Since then, WaveMode was developed in collaboration with researchers from ZHAW and EPFL to provide a new, simpler implementation of photothermal off-resonance tapping that does not rely on background subtraction for feedback control but uses the amplitude reduction of the cantilever trajectory when interacting with the sample surface.
WaveMode converts the AFM cantilever from a simple detector of tip-sample interactions into a combined detector and actuator. AFM cantilevers are well-know for their capability to serve as fast actuators. Paired with photothermal excitation, the AFM cantilever is used to provide the oscillatory relative motion between AFM cantilever tip and sample surface that is typical for off-resonance modes. The combined high bandwidth of both the cantilever and the photothermal excitation allow overcoming the main speed-limiting factor in conventional off-resonance modes – the z-scanner that is typically used to generate the relative oscillatory motion.
WaveMode provides force control down to the pico-newton level throughout the imaging process. The amplitude reduction observed upon tip-sample interaction relates to the interaction force between sample and surface and thus allows tailoring the interaction force according to the requirements of the sample. Moreover, it significantly reduces lateral forces acting on the sample during imaging. This allows also imaging samples that are otherwise difficult to image due to their displacement during the imaging process.
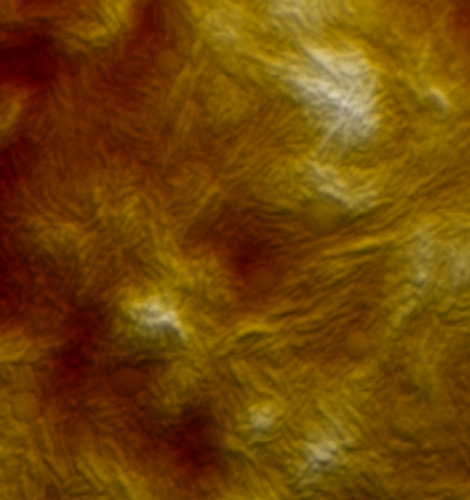
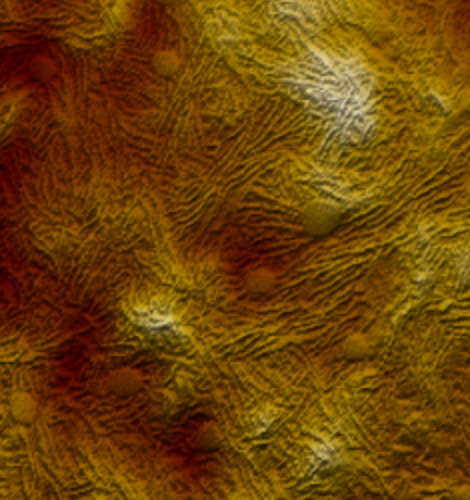
Boosting off-resonance imaging speed
WaveMode brings off-resonance imaging to new levels of performance. While the ramp rate of classical off-resonance imaging is limited by the dynamics of the z-piezo, WaveMode overcomes this limitation by using the cantilever as actuator. This leads to an increased actuation bandwidth and enables faster imaging speeds without compromising image size or resolution.
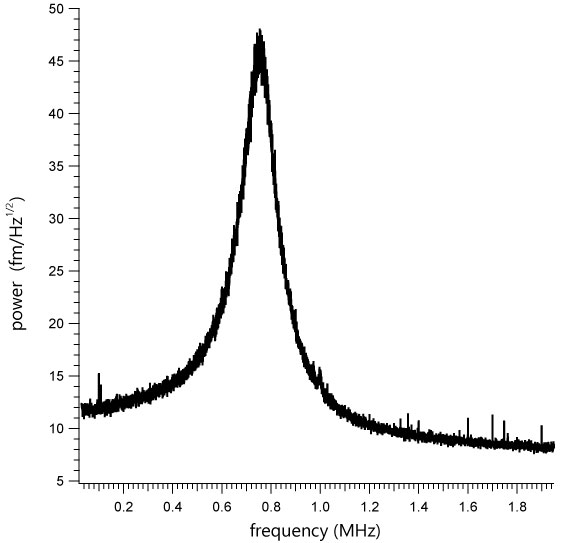
SBR-PMMA blend recorded in WaveMode at 20 kHz actuation frequency. Image size: 6x6 µm2. Recorded at 10 Hz line rate.
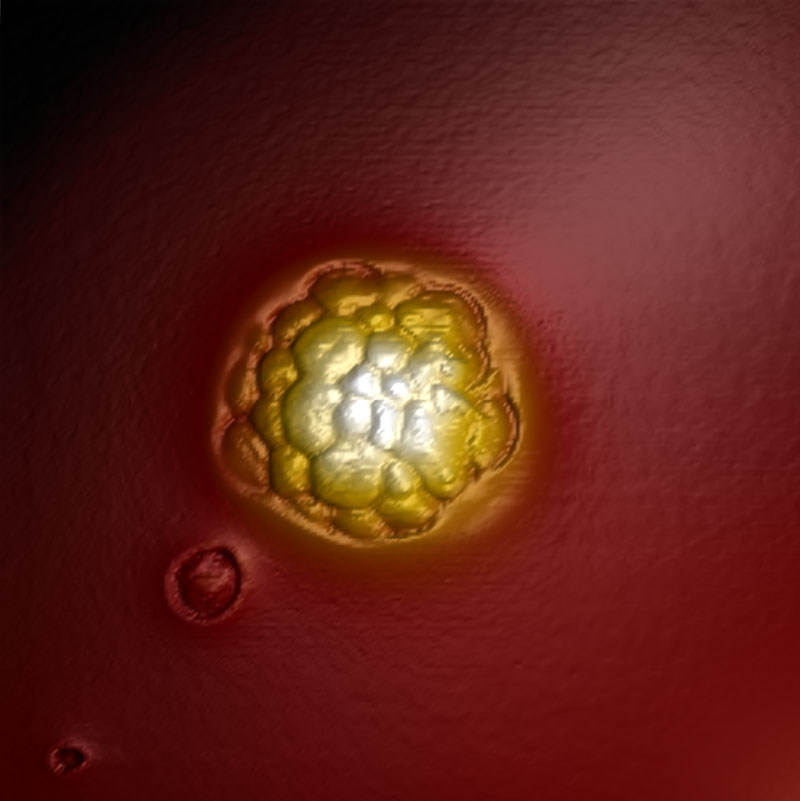
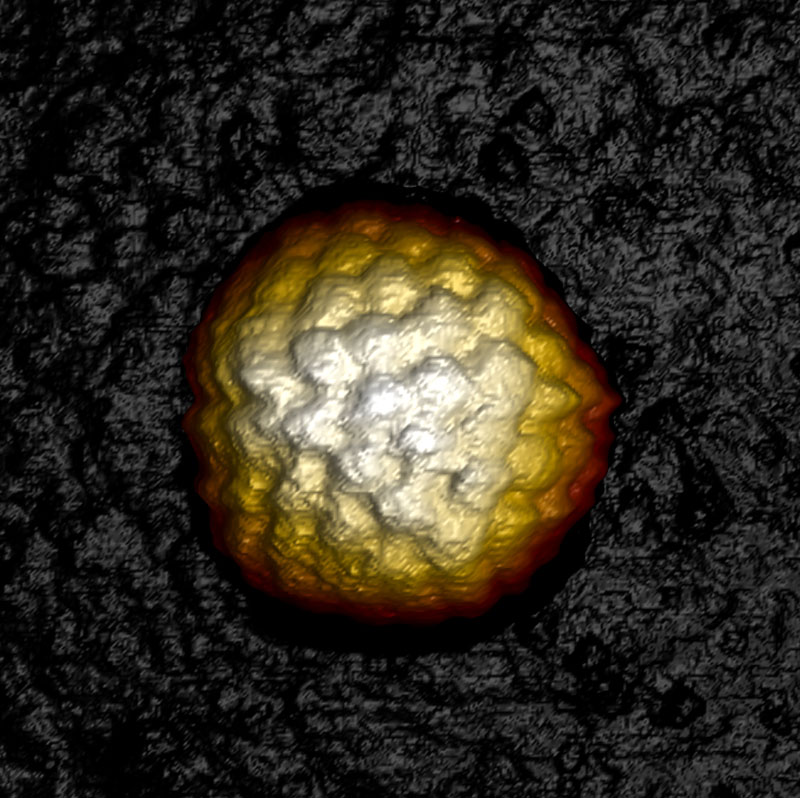
Herpes simplex virus 1 capsid
Herpes simplex virus 1 (HSV-1) capsid imaged in WaveMode at 10 kHz ramp rate resolving individual capsomeres. Image size: 230 x 230 nm2, z: 60 nm. Sample courtesy: Alex Evilevitch, Lund University.
Assembly of DNA tripods
Assembly of DNA tripods structures: time-lapse imaging of DNA tripods assembling into a hexagonal structures. Image size: 250 x 250 px2, 5 Hz line rate, 50s/frame, image width: 1 µm
Sample courtesy: Cem Tekin, Prof. Maartje Bastings, Programmable Biomaterials Laboratory EPFL.
Image courtesy: Veronika Cencen, Prof. Fantner group, Laboratory for Bio- and Nano-Instrumentation EPFL.
Straight and twisted mouse tail collagen fibrils
WaveMode image of straight and twisted mouse tail collagen fibrils measured in tris-buffered saline (TBS). The topographic image nicely shows the 67 nm banding that is typical for collagen. Measurement was performed with WaveMode in Nanosurf Studio. Scan size: 5 µm @ 500 x 500 px, scan speed 2.5 Hz line rate and 20 kHz WaveMode oscillation frequency.
Life science
DriveAFM for biology and life sciences
The DriveAFM plays out all its advantages when it comes to biological applications. The full motorization allows adjusting the lasers and photodetector, and navigating the sample, without interfering with a temperature-controlled environment. CleanDrive excitation provides reliable and clean cantilever tuning in liquid environments. The insensitivity of the CleanDrive towards environmental changes and the sensitivity of small cantilevers facilitate imaging of delicate samples over long periods of time with ease.
Seamless integration of the DriveAFM with an inverted optical microscope allows transmitted light and fluorescence microscopy to be combined with AFM imaging and force spectroscopy. The light sources‘ wavelengths used for CleanDrive (785 nm) and the deflection detection (840 nm) were selected to avoid interference with biological samples and to make fluorescence imaging possible.
The DriveAFM comes with a new line of accessories for biological applications from single-molecule investigations to live cell observations. It includes the new Petri dish holder and the new 150 µm z-actuator, which is essential for cell adhesion experiments. Both sample holders are designed to maintain biological samples at physiological temperature and to be ultimately converted into 2-chamber cell incubators.
The DriveAFM is ready for research that goes beyond conventional imaging. FluidFM® and PicoBalance expand the capabilities of the DriveAFM with multifunctional hollow cantilevers and cell mass measurements.
AFM and optical super resolution
AFM topography and superimposed STED image (SiR-actin) of mouse fibroblasts in cell culture medium. Learn more in this paper: How did correlative atomic force microscopy and super-resolution microscopy evolve in the quest for unravelling enigmas in biology? - Nanoscale (RSC Publishing)
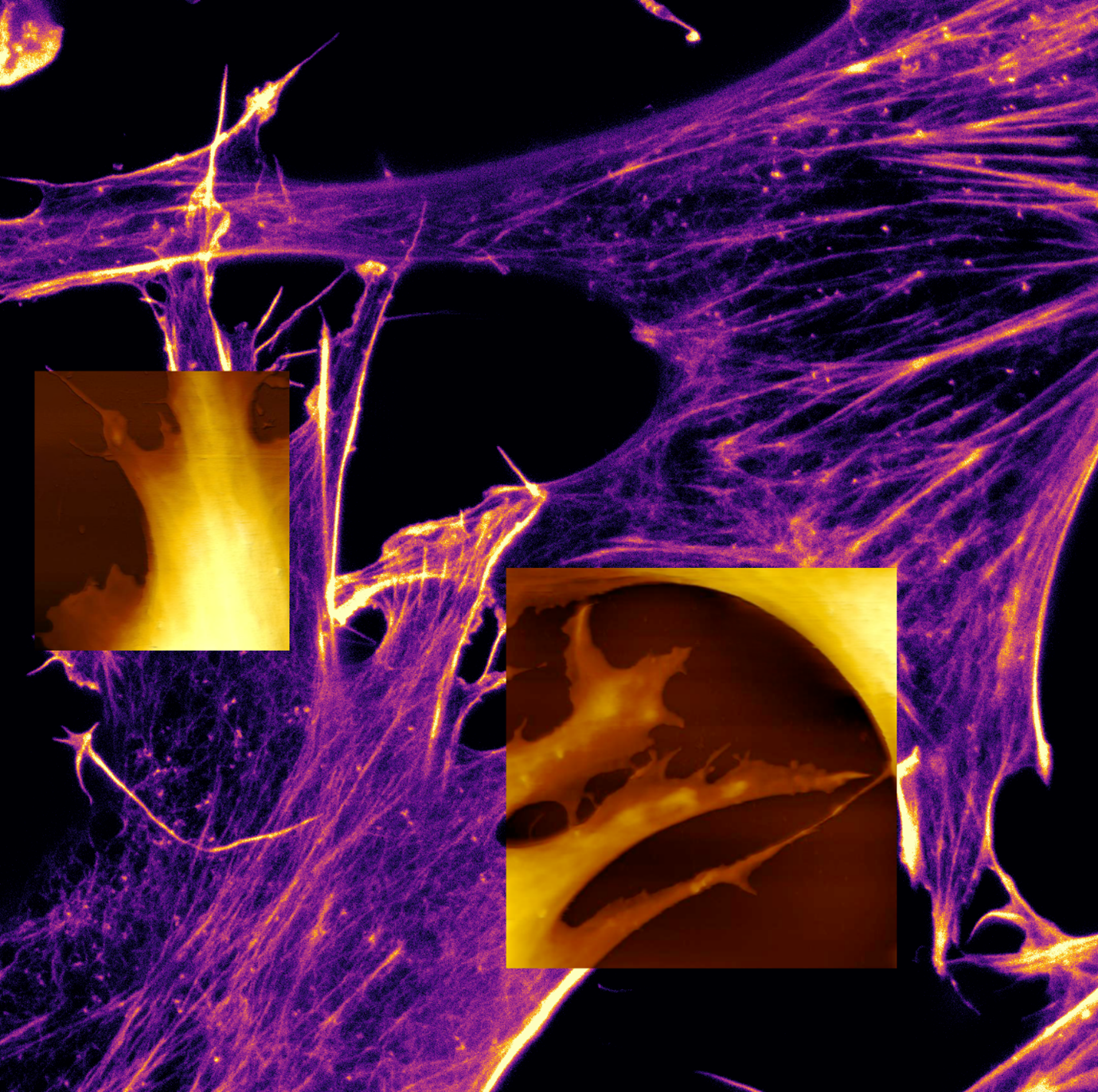
Embryonic mouse fibroblasts
AFM topography image of immortalized embryonic mouse fibroblasts in cell culture medium.
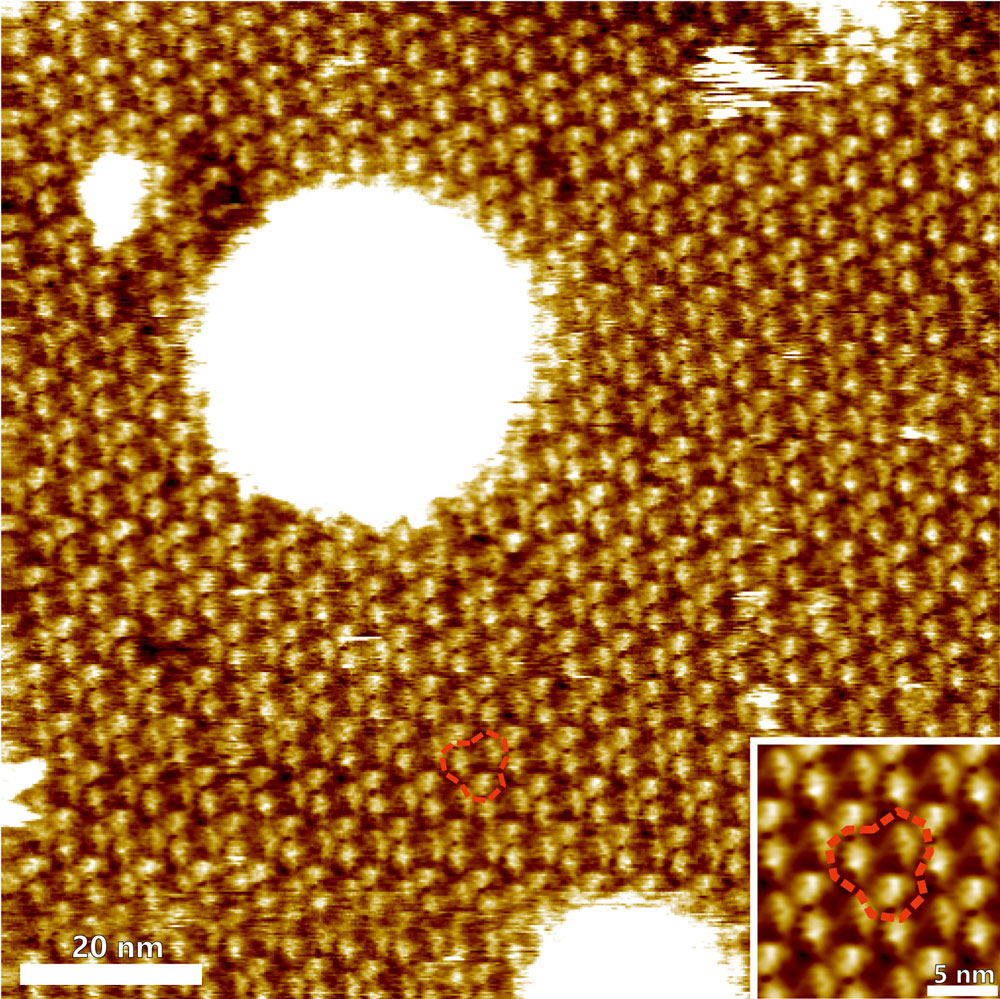
Trimeric arrangement of bacteriorhodopsin (BR) proteins and substructures
High-resolution topography image of the cytoplasmic surface of purple membrane from Halobacterium salinarum. The topography clearly resolves the trimeric arrangement of bacteriorhodopsin (BR) proteins and substructures within the BR molecules. Inset: correlation average of BR trimers. The red dashed lines indicate a BR trimer.
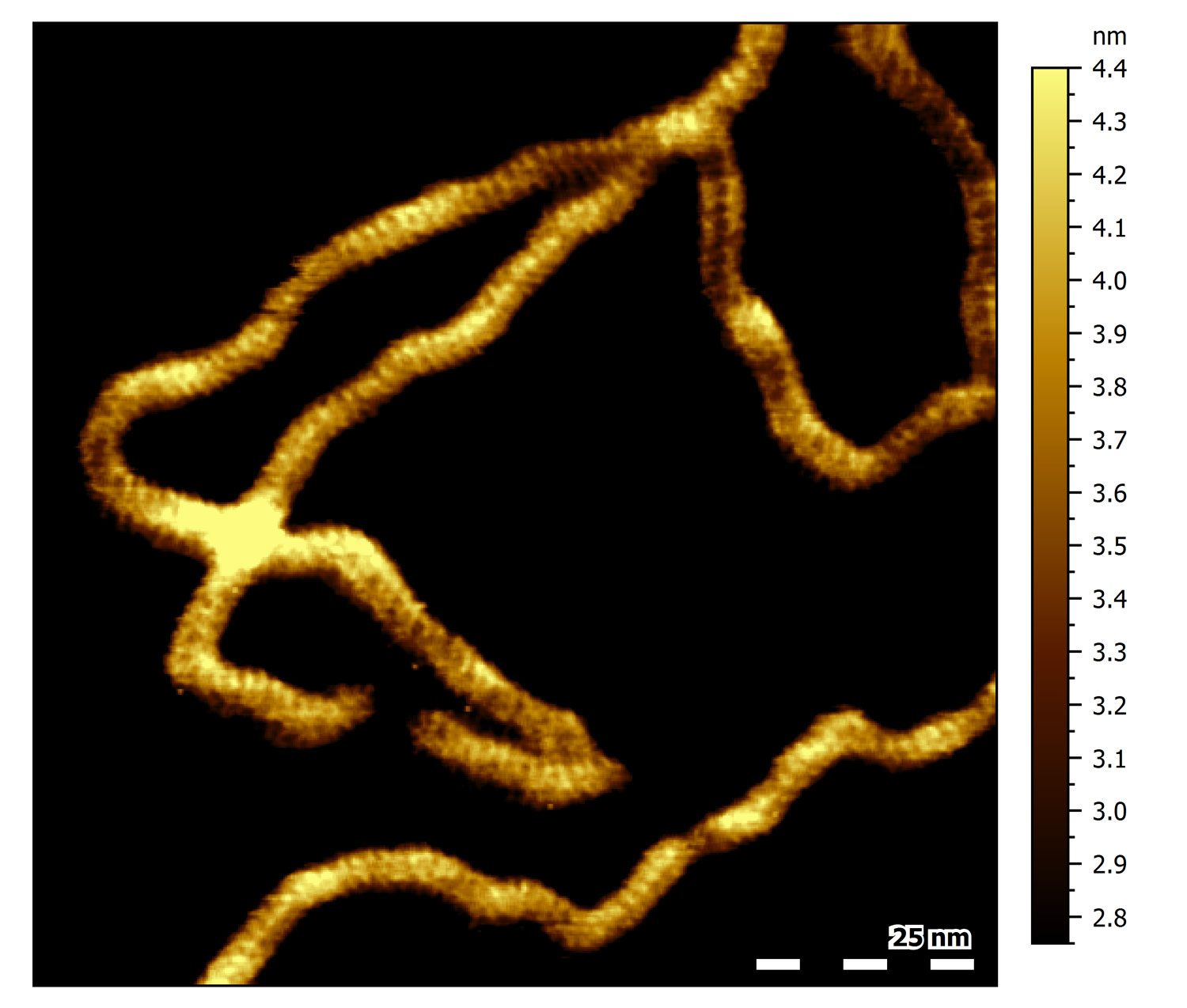
DNA major and minor grooves
High-resolution topograph of double-stranded DNA (dsDNA) adsorbed to mica in buffer solution. Several dsDNA strands can be observed. All of them show a characteristic periodic pattern. Click on the image for a close-up: the black line indicates the location of the cross section shown in the inset. The average spacing between every second-next groove in the section corresponds to 3.4 nm, the characteristic pitch distance of a helical turn of B-DNA. The valleys in the section correspond to the major and minor grooves found in dsDNA.
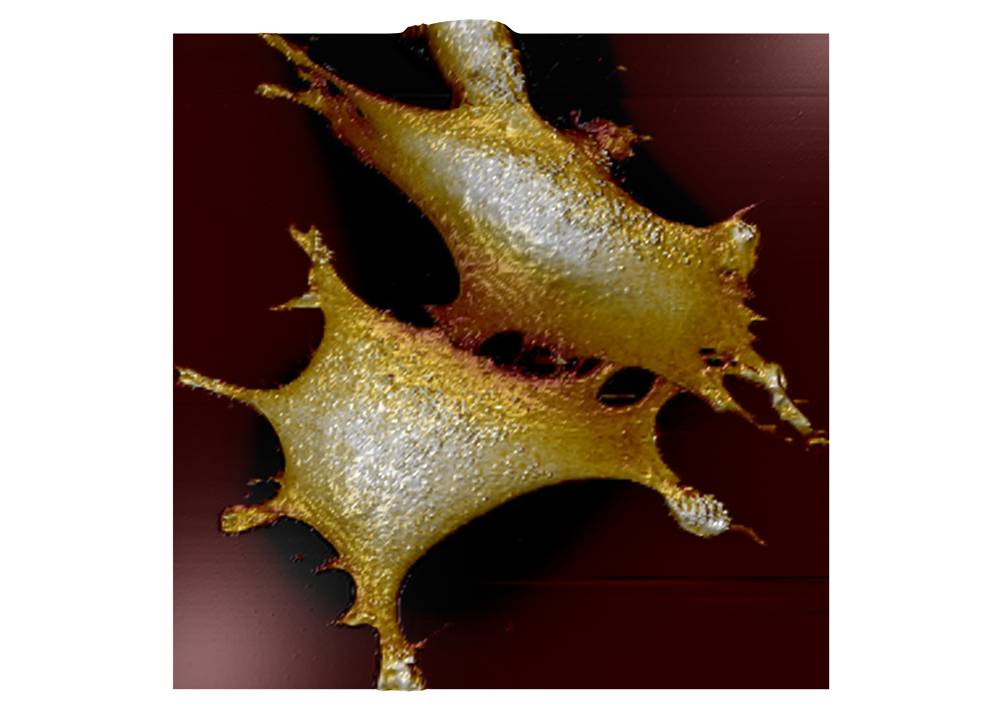
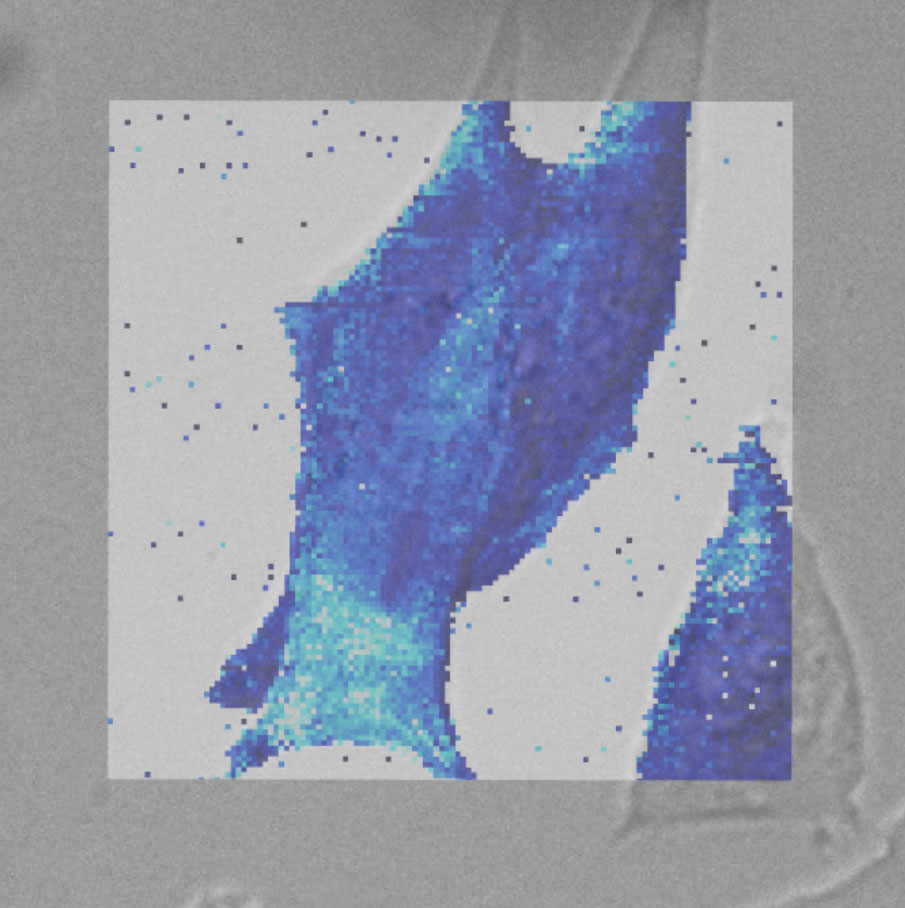
Force map of live fibroblast cell
Force map of live fibroblast cell in cell culture medium at 37°C. The force map is superimposed on the DIC image of the cell.
Seamless integration of the DriveAFM with inverted optical microscopes allows transmitted light and fluorescence microscopy to be combined with AFM imaging and force spectroscopy. The wavelengths of the light sources used for CleanDrive (785 nm) and deflection detection (840 nm) were selected not to interfere with biological samples and fluorescence imaging.
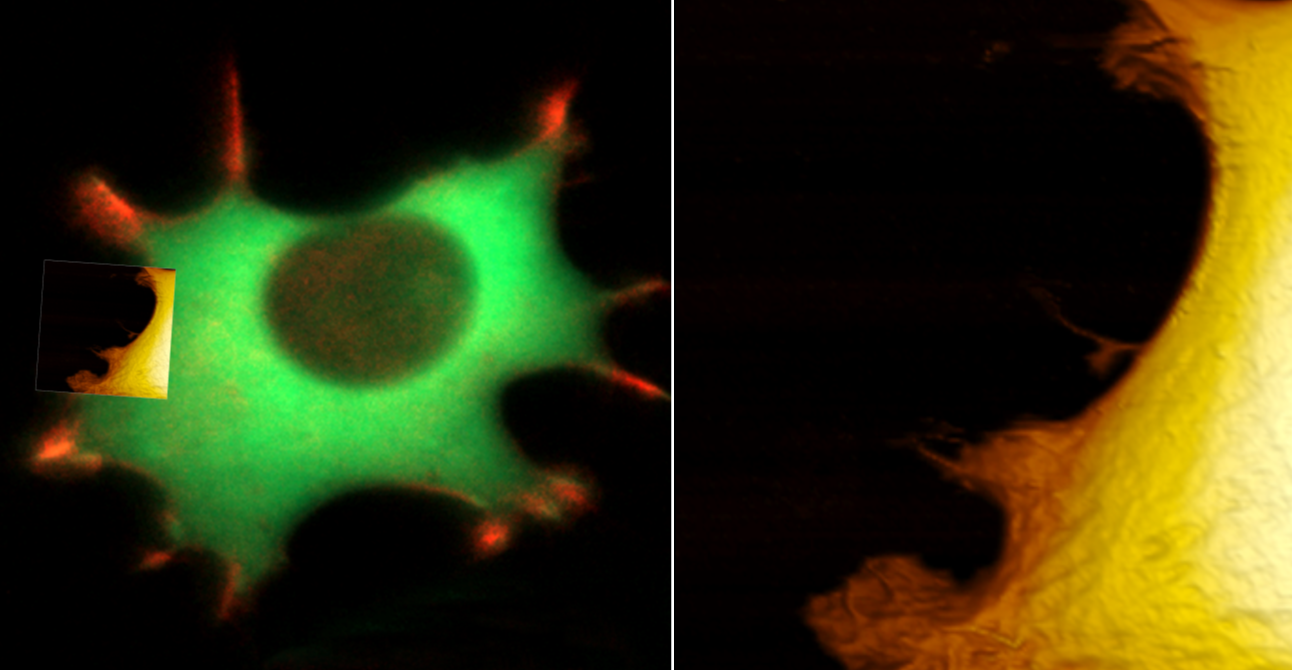
Combining the DriveAFM with optical light microscopy for biological research
Live fibroblast imaged with inverted light microscope and AFM in cell culture conditions. Left, cell with genetically encoded fluorescent makers (actin-mcherry, gfp-paxilin). Right, AFM image, 10 µm width.
FluidFM® ready, PicoBalance ready, ANA ready
The DriveAFM is ready for research that goes beyond conventional imaging. FluidFM, PicoBalance and ANA expand the functionality of the DriveAFM with multifunctional hollow cantilever, cell mass measurements and measurement automation.
Materials science
DriveAFM for materials science
The DriveAFM combines performance and a wide range of applications important for material science research. Its unique direct drive tip scanner technology paired with CleanDrive are key for fast and stable operation in air and liquid. The tip scanner design makes the performance of the DriveAFM independent of the mass of the sample under investigation also allowing measurement on heavy samples. The full motorization not only simplifies working with the system but also facilitates automated measurements addressing different areas of a sample.
Besides reliable topographic imaging, the DriveAFM also features a complete set of different modes to investigate the nanoelectrical (e.g. C-AFM, KPFM, or PFM) or nanomechanical properties of your sample. The universe of accessories available for the DriveAFM offers extended functionality such as heating or cooling the sample, applying a variable magnetic field, detecting low electrical currents or investigating with in situ AFM imaging the changes taking place on electrodes during electrochemical processes.
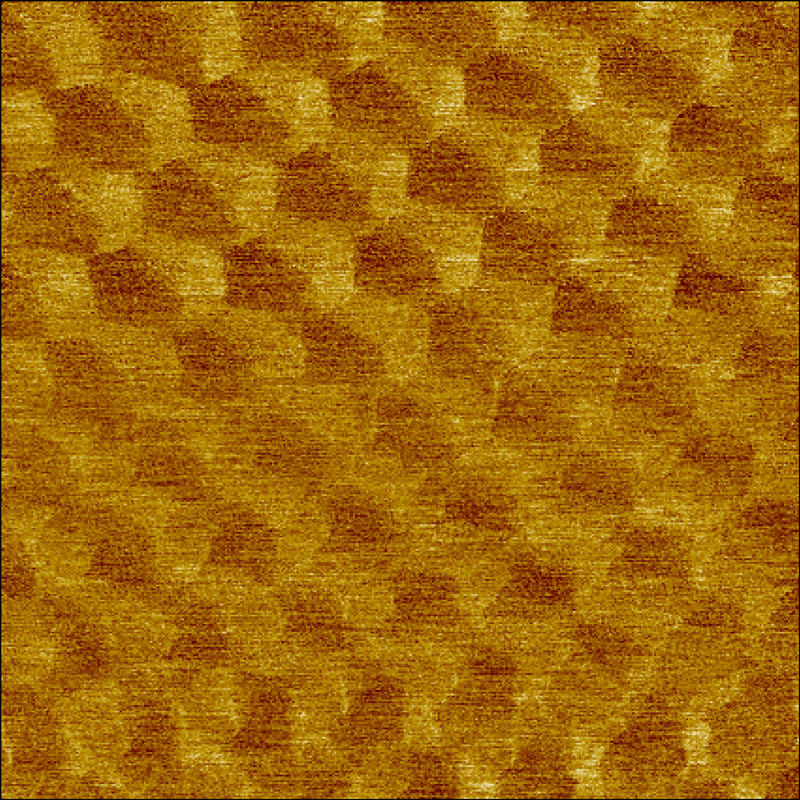
Moiré super lattice of twisted graphene on hBN
Moiré super lattice of twisted graphene on hBN imaged in force modulation mode on the contact resonance frequency. (A) phase image with scan size of: 190 x 190 nm2
HOPG topography in static mode
HOPG topography recorded in static mode.
XY-range: 3 nm, z-range 195 pm.
Distance between visible atoms: 0.246 nm.
No Fourier or Gaussian filtering applied.
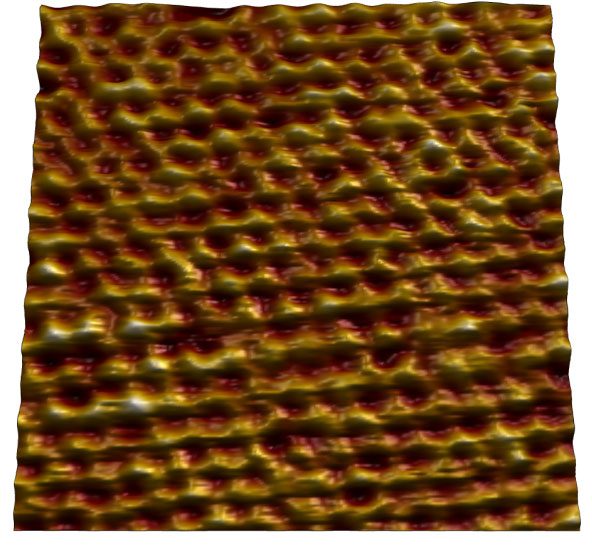
Mica
Atomic lattice of mica imaged in air. Image size: 7 nm.
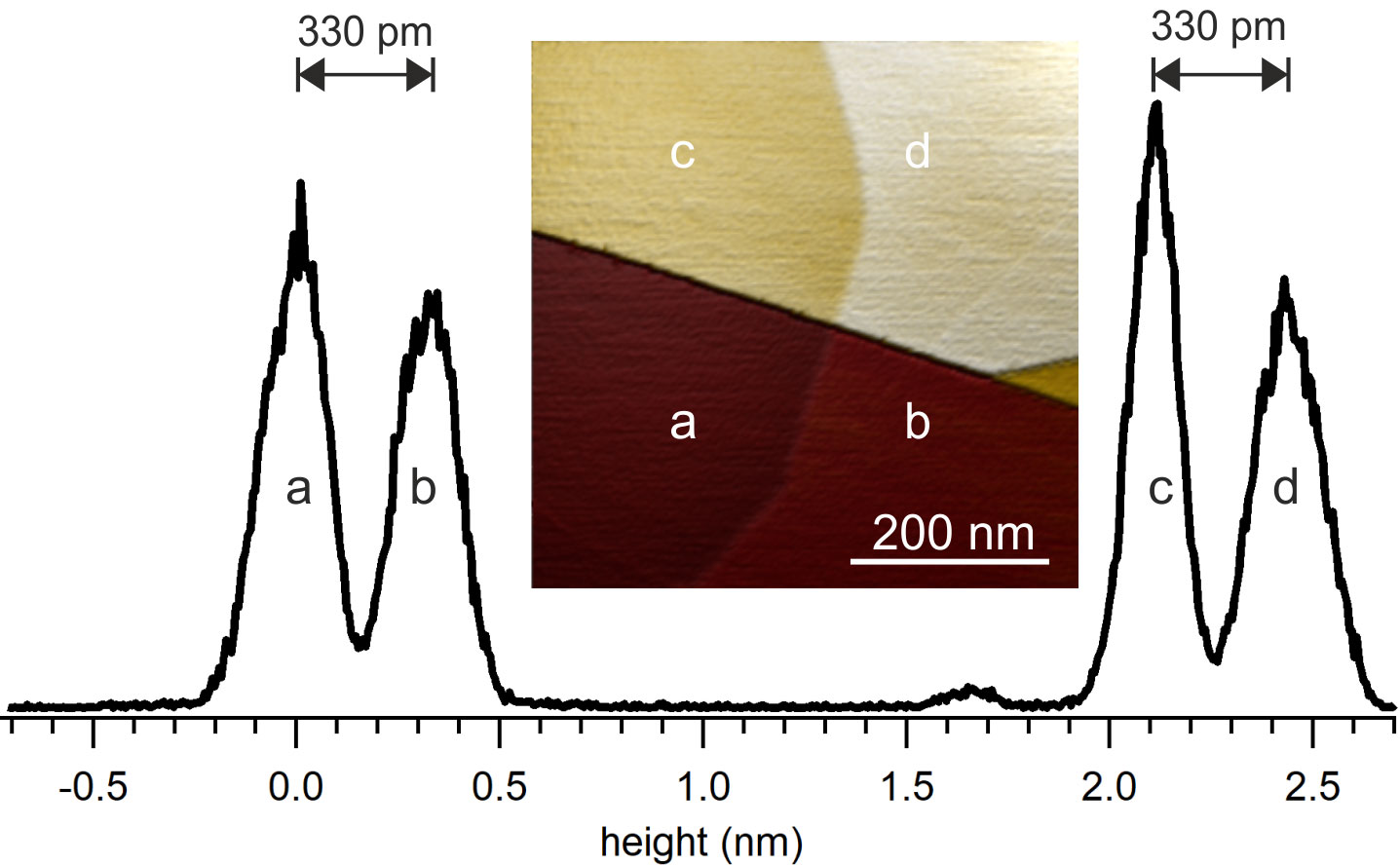
Topography of an HOPG surface imaged in air
Topography of an HOPG surface imaged in air: the surface shows different steps between different graphite layers. Height histogram of the HOPG surface shown on the right. Image size: 500 nm. The spacing of two neighboring peaks in the histogram corresponds to 330 pm, the expected height of a graphite layer.
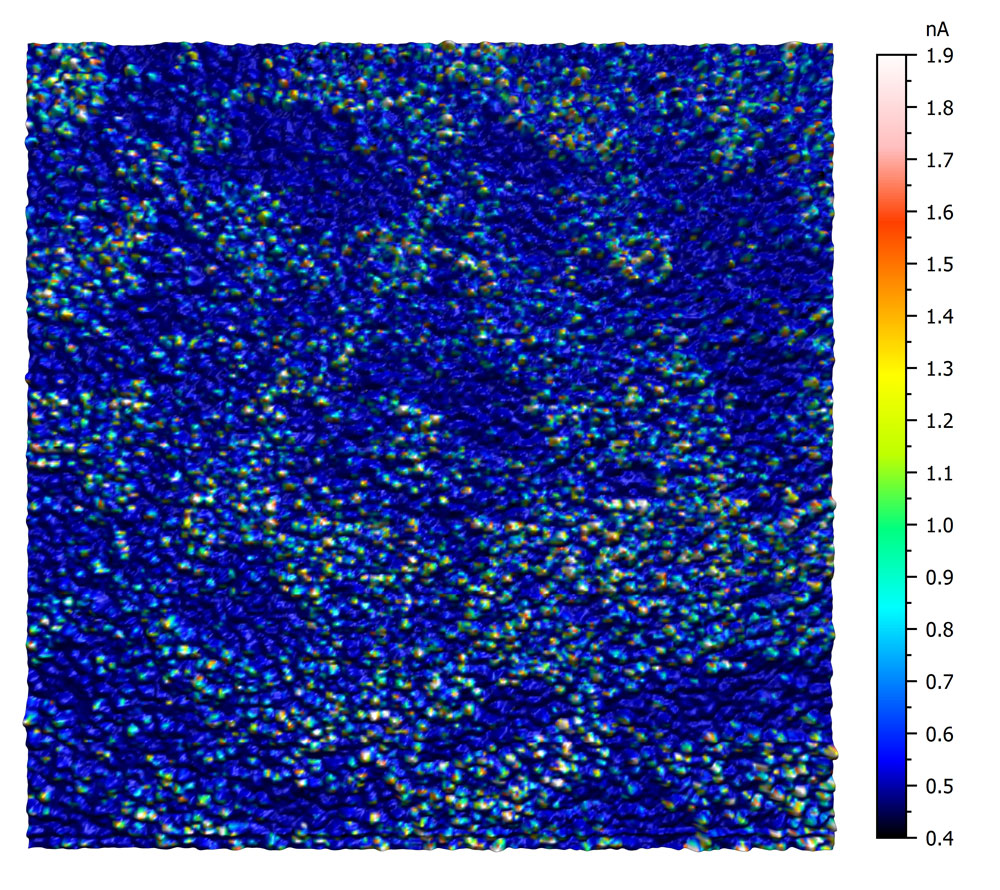
Conductive AFM on ITO
This conductive AFM measurement on ITO reveals the heterogeneous conductivity of an untreated ITO surface. The image was recorded using an EFM cantilever with a tip bias of 5mV. The current was measured using the C-AFM sample holder. Color scale 1.5 nA.
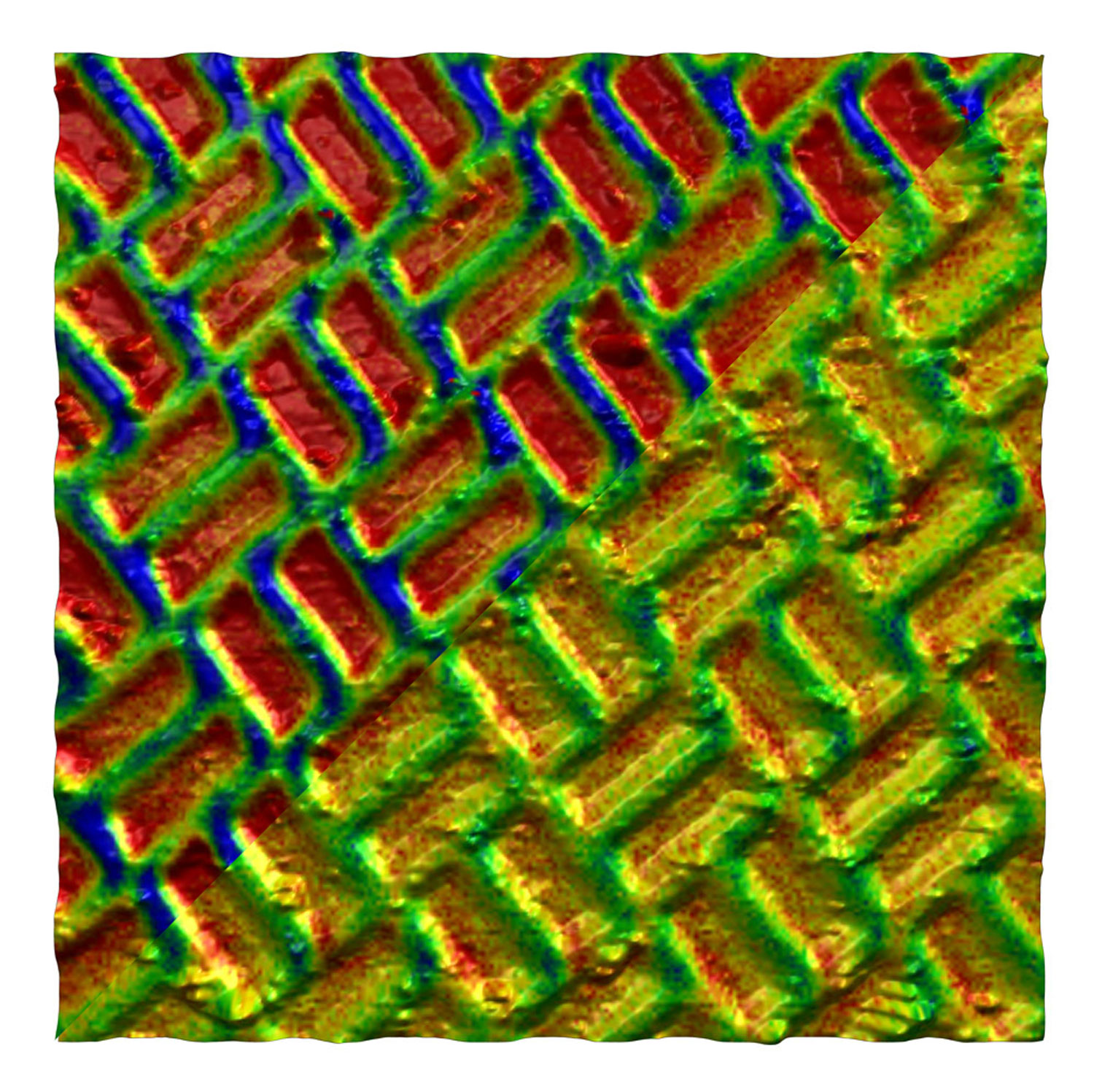
PS-Pb-PS triblock copolymer thin film on mica imaged at 20 Hz line rate
This image shows the phase (top) and topography (bottom) of an unannealed PS-PB-PS triblock copolymer thin film on mica imaged at 20 Hz line rate using an USC-F1.2-k7.3 cantilever.
MFM images of a Shakti lattice
MFM images of a Shakti lattice while applying different in-plane magnetic fields (top: 200 mT, bottom: 50 mT). The images represent the 3D topography of the lattice, and the color scale overlaid corresponds to phase shift induced by magnetic interactions.
Image size: 8 µm, phase signal range: 1°.
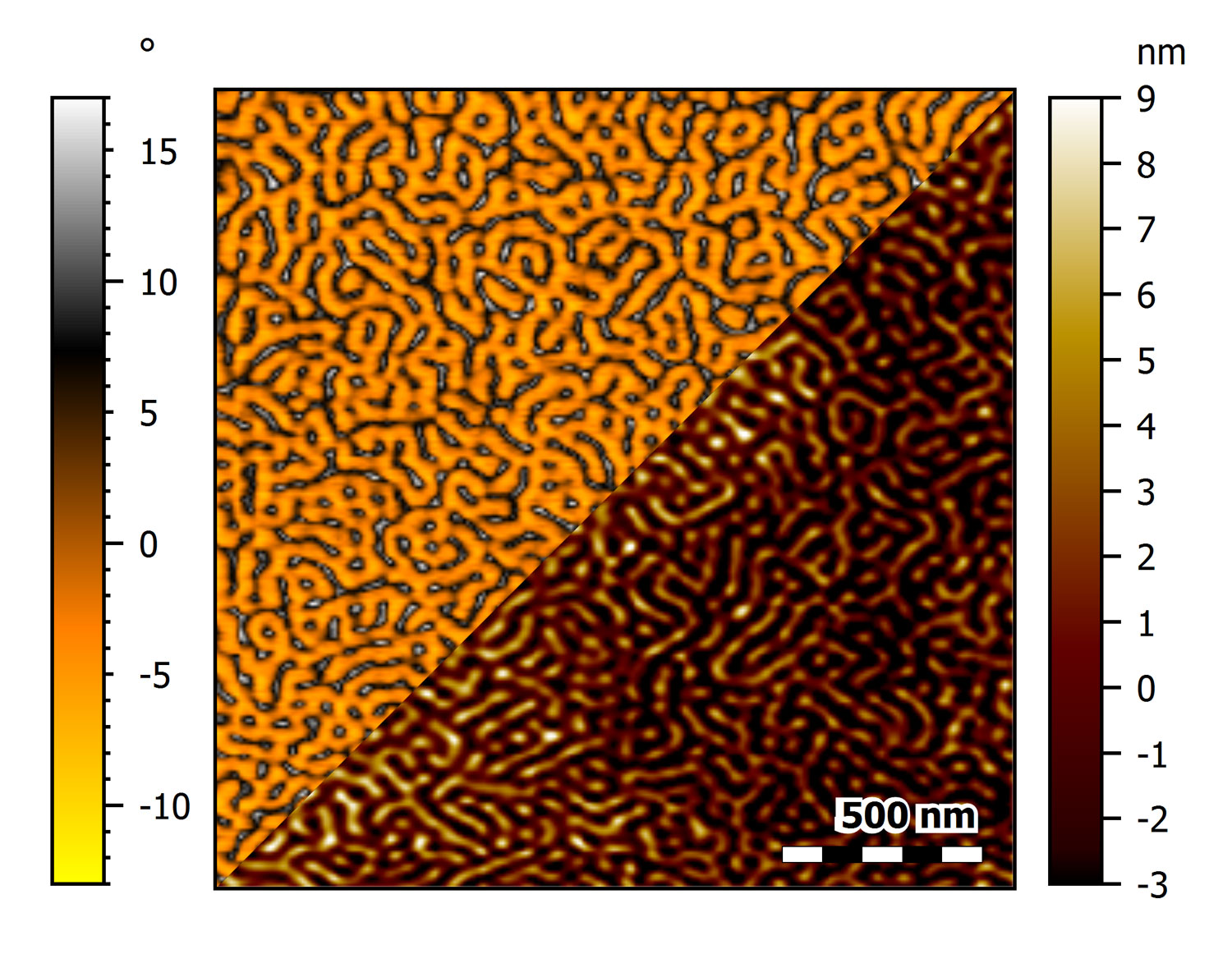
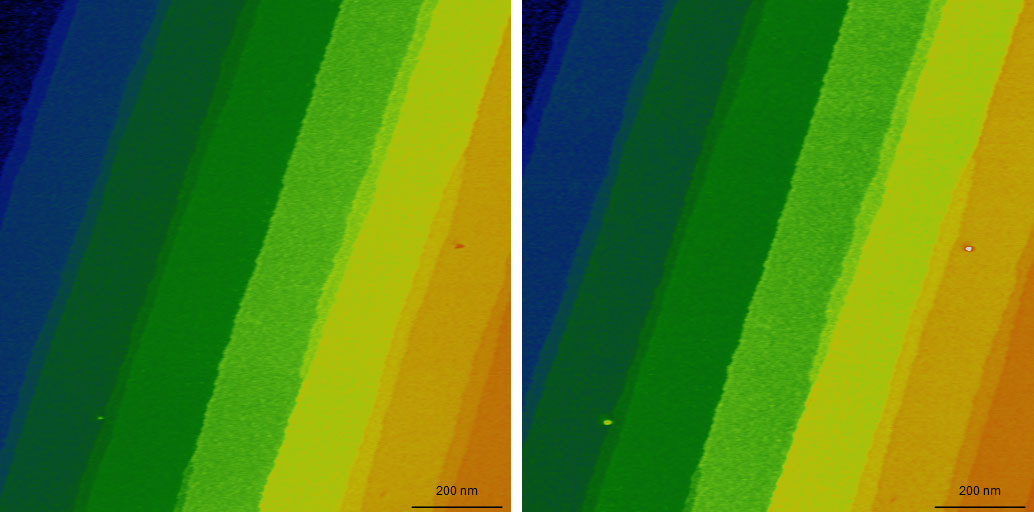
AFM topography of two images of SiC showing 1.5 nm steps on its surface
The two images were taken at the beginning and end of a series of 300 images over the course of about 14h using CleanDrive excitation. During imaging, no adjustments were made to the imaging conditions. Color scale: 12.5 nm.
Versatility without compromise
The DriveAFM offers a wide range of applications to investigate material properties. Besides pure topographic imaging, the DriveAFM also features a complete set of different modes to investigate the nanoelectrical (e.g. C-AFM, KPFM, or PFM) or nanomechanical properties of your sample. The universe of accessories available for the DriveAFM offers extended functionality such as heating or cooling the sample, applying a variable magnetic field, detecting low electrical currents or investigating with in situ AFM imaging the changes taking place on electrodes during electrochemical processes.
SMM
Scanning Microwave Microscopy (SMM)
Key benefits
- Electrical characterization of materials – dielectrics, semiconductors and metals
- High sensitivity to changes of dopant density in semiconductors
- High sensitivity to DC capacitance
- Minimal sample preparation
- Imaging of buried structures
SMM technique
Scanning microwave microscopy (SMM) is a scanning probe technique, that measures the interactions of a microwave from a sharp tip with the sample. The microwave reflection coefficient (S11 parameter), which is the ratio between the microwave power sent to the tip and the one received back after being reflected at the tip-sample contact, is used to deduce the local tip-sample microwave impedance. The microwave impedance yields information about the local capacitance from which one can deduce the dielectric constant and the dopant density. In terms of the dopant density measurement, the SMM is similar to the scanning capacitance microscopy (SCM), but offers wider range of measurements. SMM can be used for measurements of not only semiconductors, but also dielectric materials and metals, since it is not solely relying of the modulation of the depletion capacitance in the sample.

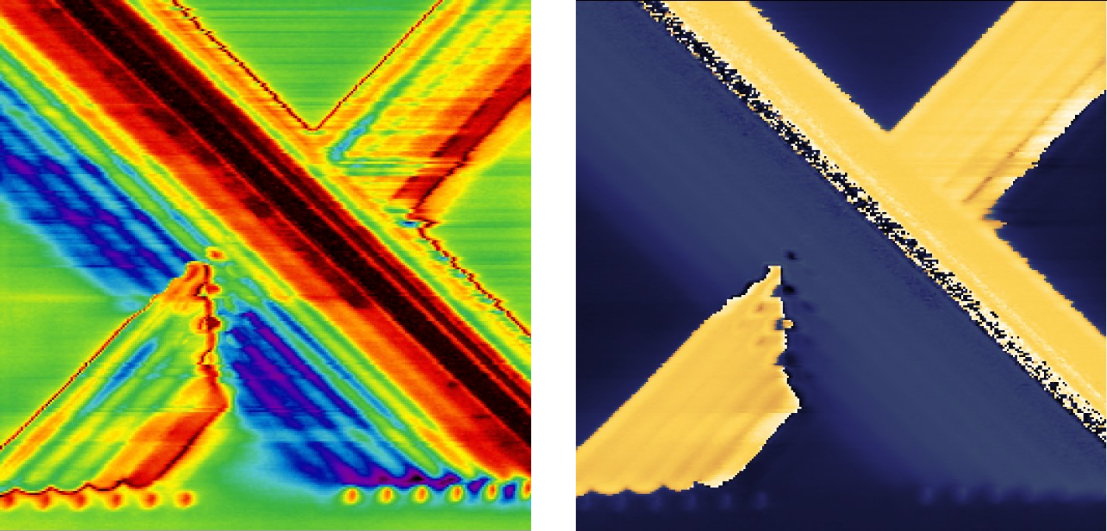
Understanding the S11 Parameter
The S11 parameter is defined as a function of the complex impedances of reference impedance (Z0) and load (ZL) – Eq.1. Impedance is a complex value and can be represented as a sum of real and imaginary parts – Eq.2. The real part of Z is resistance (R) and the imaginary part can be considered as capacitance (C) -Eq.3. Depending on the sample, resistance and capacitance are functions of conductivity, dielectric constant and carrier density.
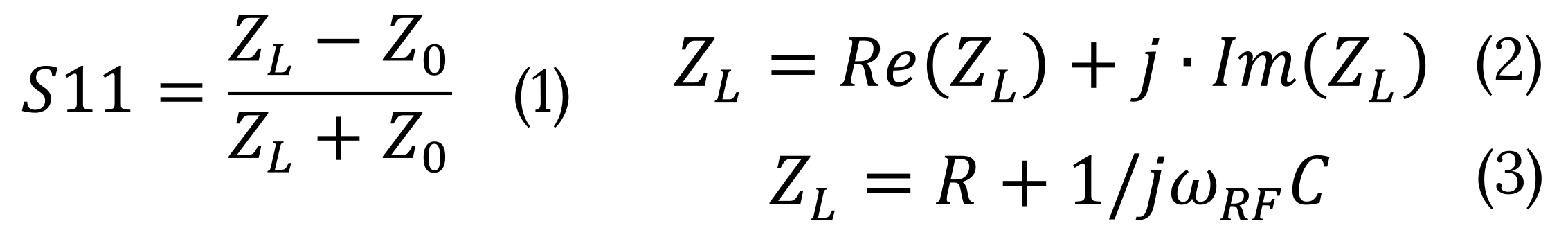
Download the SMM brochure
Hardware
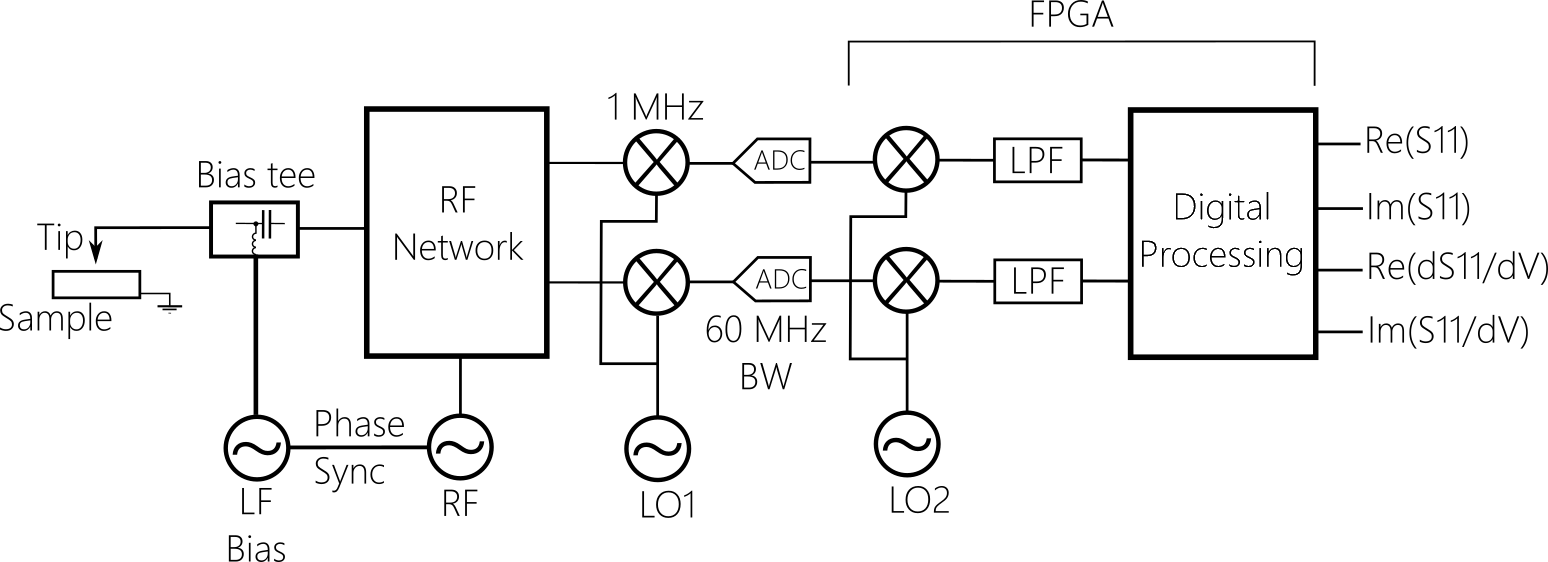
Powerful digital signal processing is analysing signal at 1 MHz, far away in frequency from 1/f noise. The signal is digitized early at the analog front-end and such typical issues of analog systems as DC-offset re-adjustment are absent in this configuration. FPGA software has sideband detection capabilities, and dS/dV (dC/dV) measurement does not require any additional hardware, e.g. lock-in amplifiers. Both S11 and dS/dV signals are digital. High working frequency around 5 GHz means better sensitivity to capacitance.
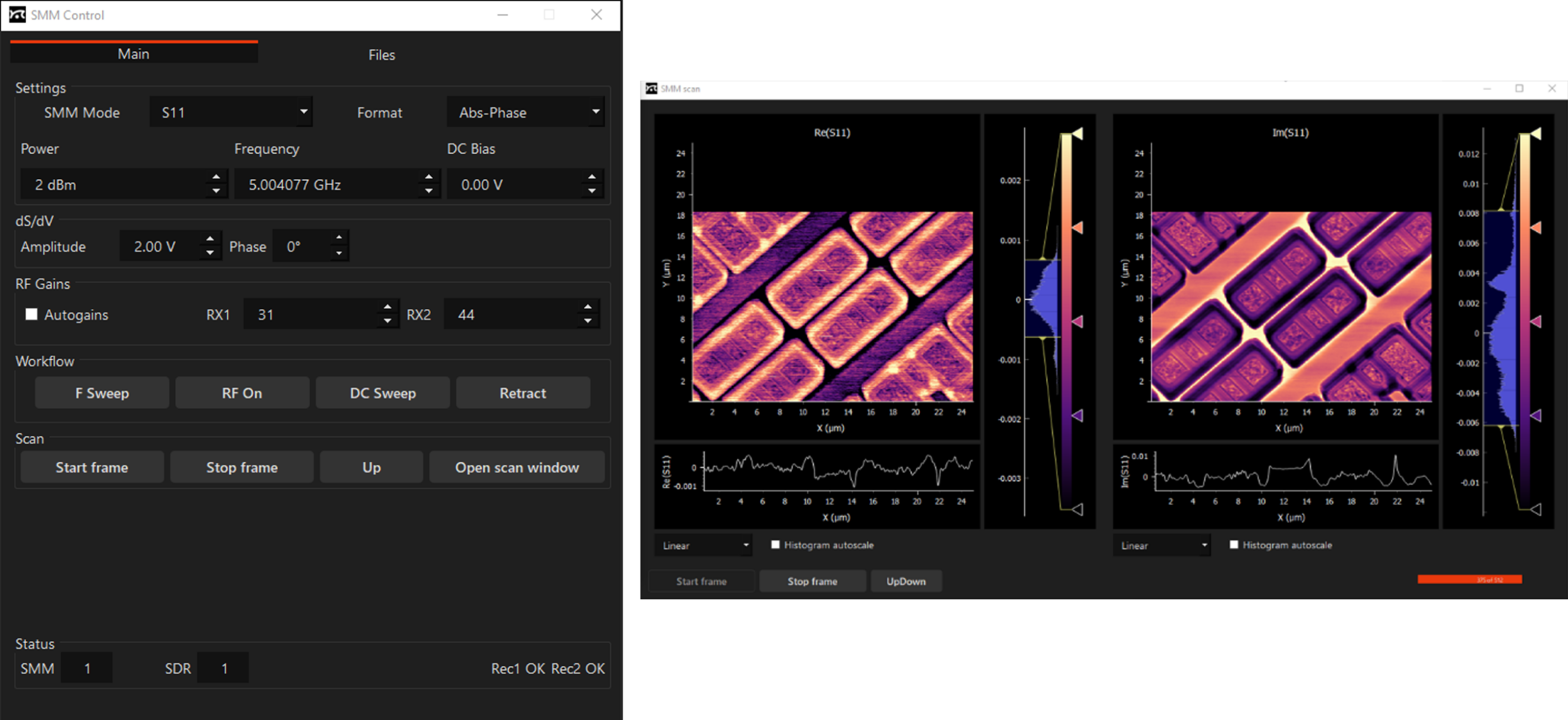
Software
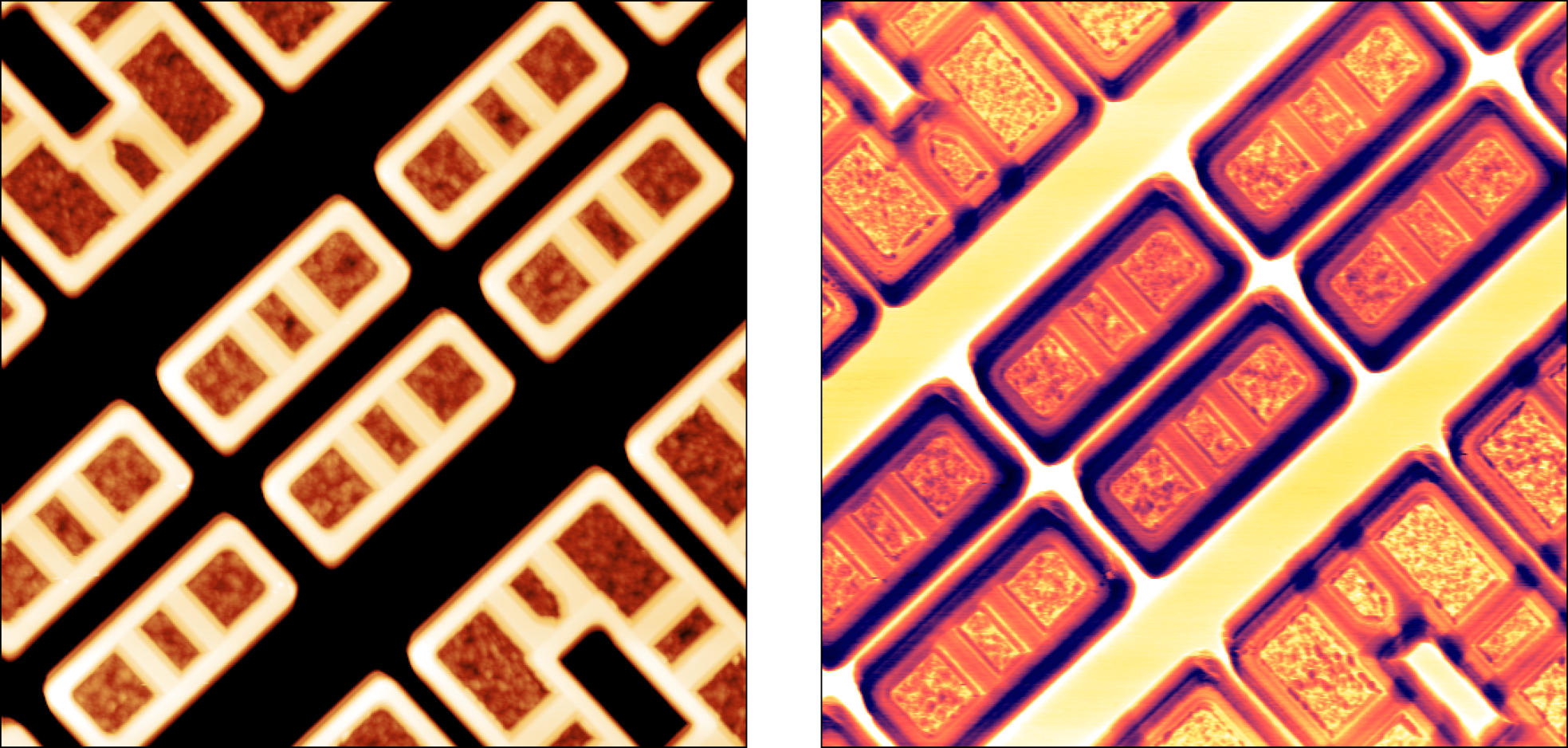
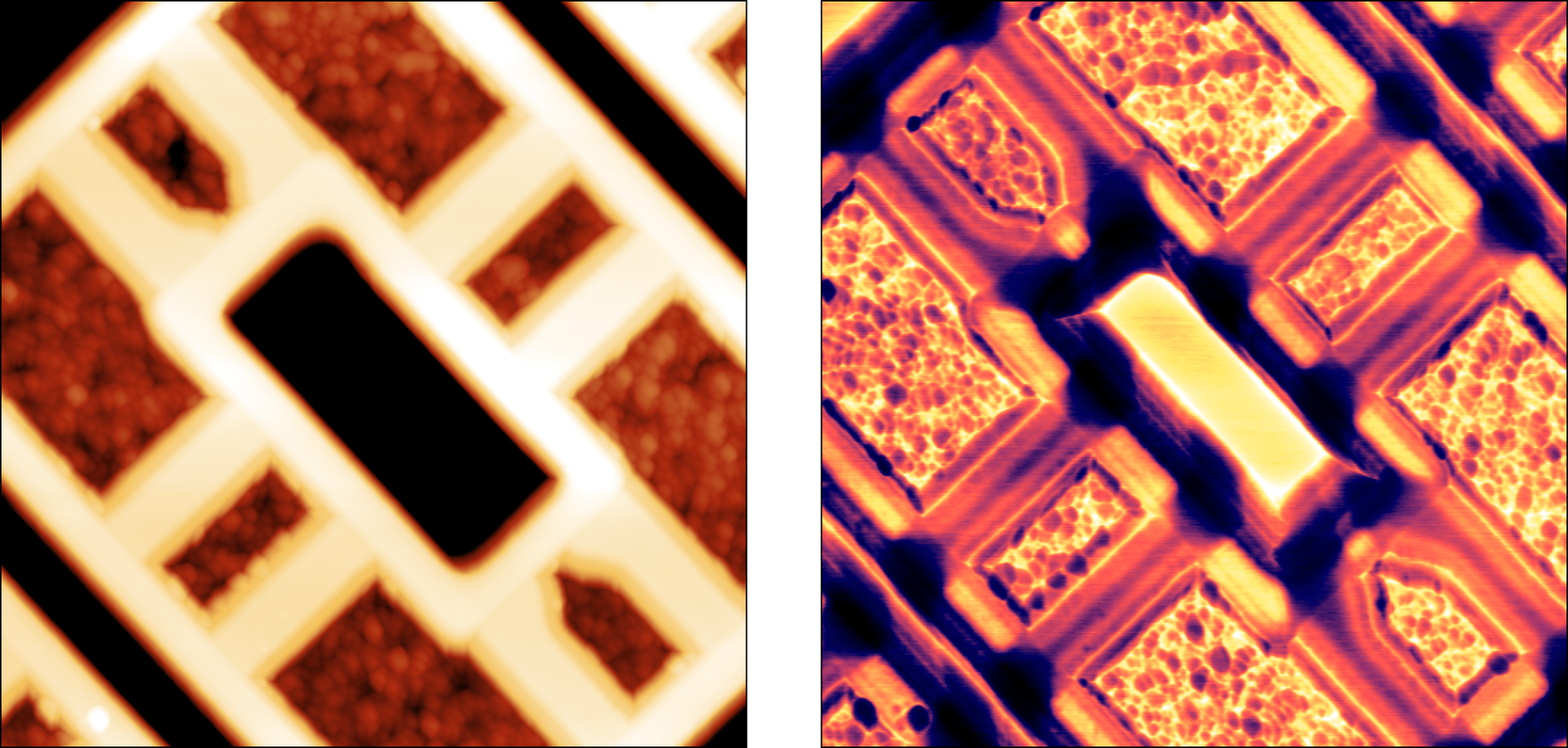
Application examples
SMM provides ultra-sensitive measurements of local capacitances. A capacitance measurement can yield material or device parameters, and it is indispensable for characterization of semiconductor materials, and electronic structures. Read in more detail about application examples in our SMM technical note and application note on Studies of MC2 capacitance standard sample by scanning microwave microscopy (SMM)
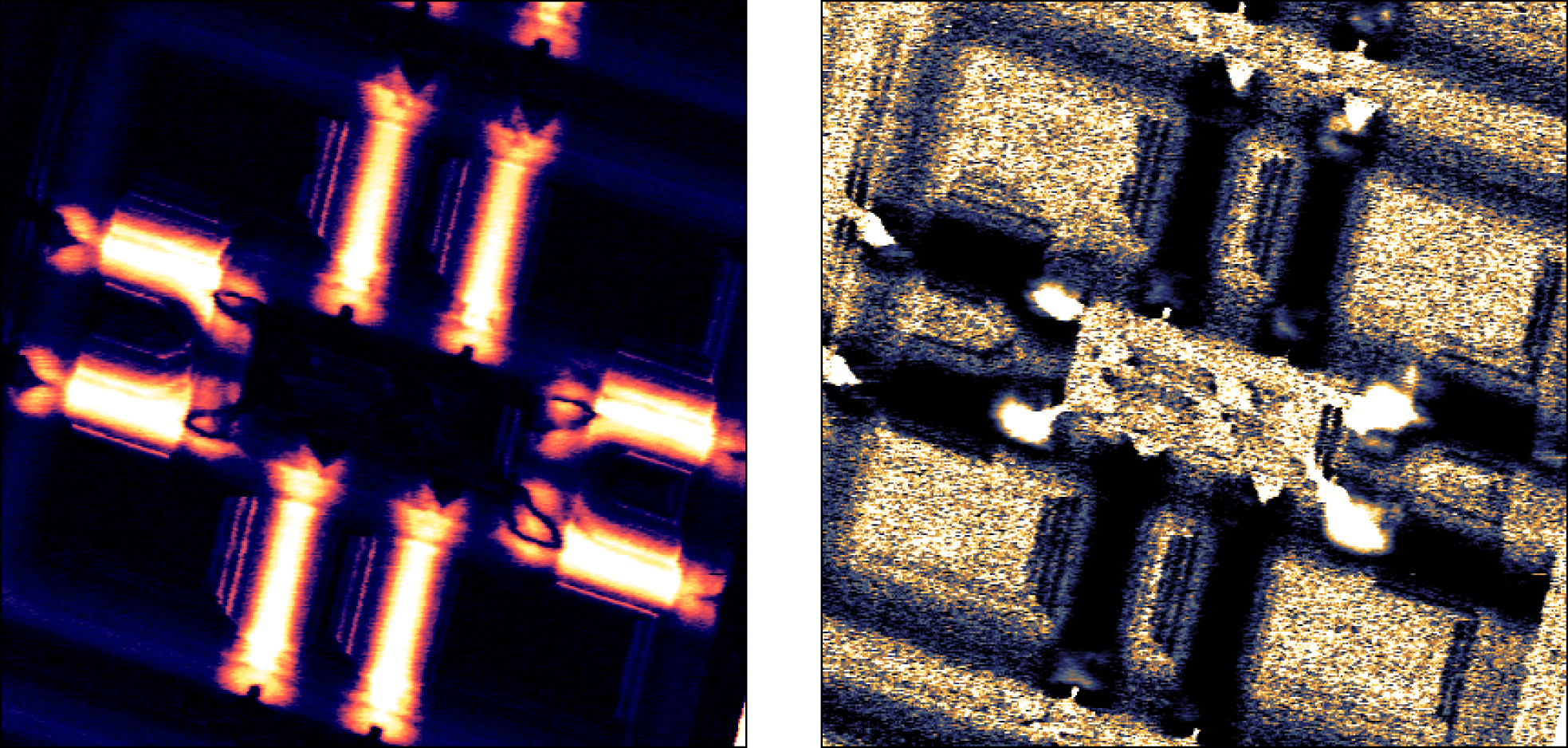


Nanosurf SMM Option
The Nanosurf SMM option is currently available for the Nanosurf CoreAFM and FlexAFM and DriveAFM.
| Specifications | |
|---|---|
| Power (dBm) | 4 |
| Frequency (GHz) | around 5.0 |
| DC noise (aF) | 0.5 |
| Doping concentration (1/cm3) | 1015 – 1020 |
| Signal down-conversion | digital |
| Software integration | yes |
| Modes | S11, dS11/dV (dC/dV, dR/dV) without external lock-in |
FluidFM
FluidFM®: The established microfluidic tool for single-cell biology and nanomanipulation
FluidFM literally brings microfluidics to the tip of an AFM cantilever. This way, it combines the microfluidics with the force sensitivity and positional accuracy of an AFM allowing a range of exciting applications in single-cell biology and nanoscience.
Nanosurf has a long experience providing AFM systems with FluidFM having launched the FlexAFM with FluidFM in 2011 together with Cytosurge. This integration of FluidFM on Nanosurf platforms has grown over time and now the FluidFM technology is available for Nanosurf's FlexAFM, CoreAFM and of course the flagship DriveAFM platform.

A tool for research pioneers
- A tool to conduct original research at the frontiers of science from bacterial adhesion to cell-cell interaction and from spotting to injection and extraction
- A solution with optical, force, and fluidic control, with full software integration on DriveAFM
- FluidFM can also be combined with PicoBalance to measure the mass of cells and microparticles picked up by the FluidFM probe
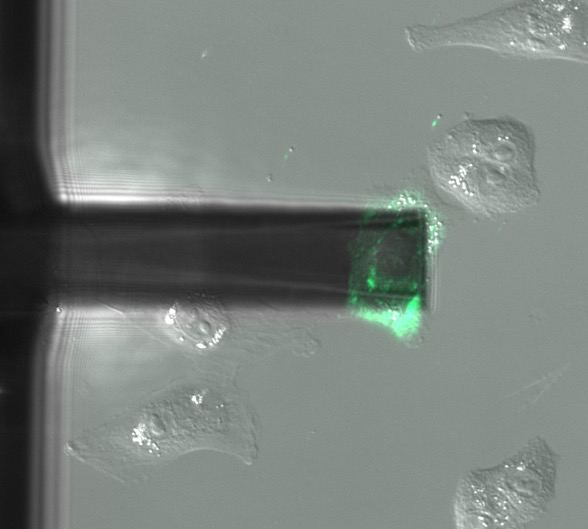
Nuclear injection
DAPI is a fluorescent marker that binds into the minor groove of DNA. Since a cell's DNA is present in the nucleus, it is a commonly used nuclear stain agent. Before conventional labelling, cells are fixed and the membrane permeabilized to allow the dye to enter the cells. Due to the high required concentration, it is conventionally not used for living cells. In the experiment below, FluidFM is used to directly inject the nucleus of living cells with DAPI. DAPI's excitation maximum is at 358 nm and its emission is in the blue part of the light spectrum (461 nm). Sample courtesy: Dr. Nico Strohmeyer, Biophysics group, Prof. D.J. Müller, ETH Zurich
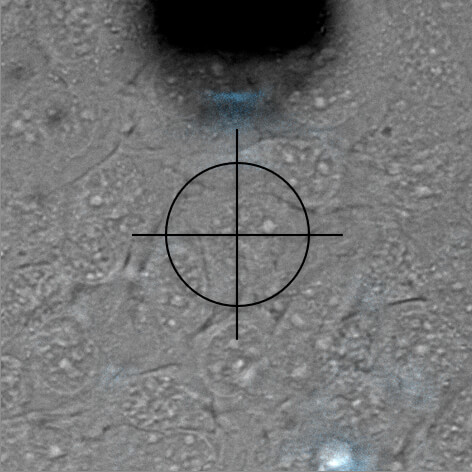
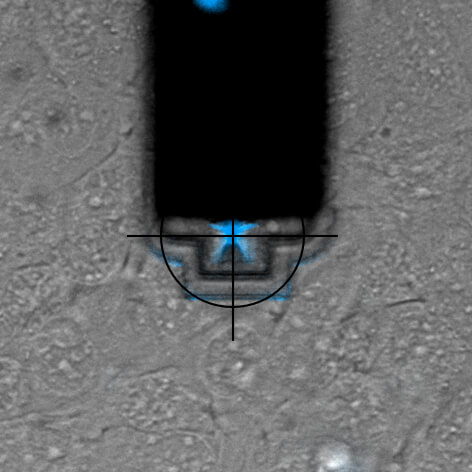
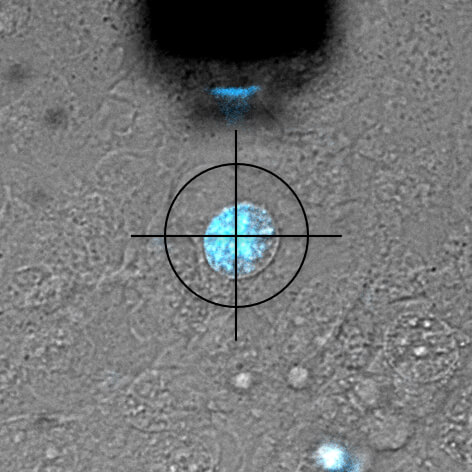
Single-cell force-spectroscopy
Single-cell force-spectroscopy studies are genarally hampered by the relatively slow pace at which a statistically relevant amount of data can be obtained. FluidFM® can dramatically increase the amount of data that can be recorded in a single day: By applying underpressure to the system, FluidFM® cantilevers can attach to a selected cell within seconds. Likewise, the probe can be immediately re-used after each adhesion experiment by releasing the detached cell from the probe through the application of overpressure. The maximum cell adhesion forces that can be measured using FluidFM are in the range of 500 pN to 1600 nN. The latter value represents a one-order of magnitude increase compared to conventional AFM approaches.
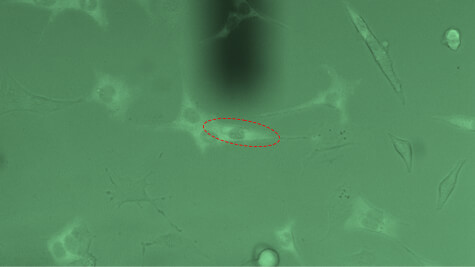
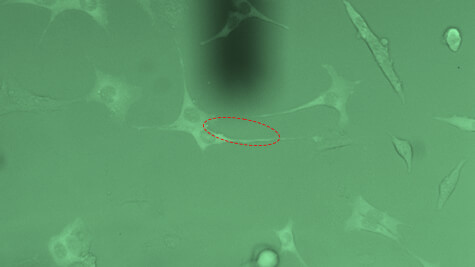
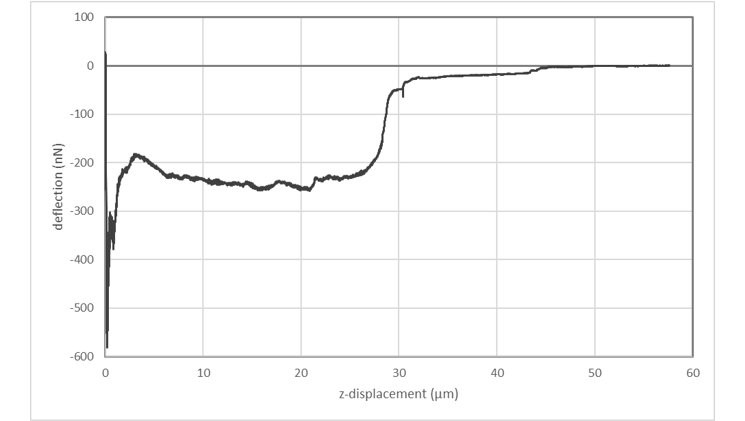
Innovative and intuitive handling
In the Nanosurf Studio software, FluidFM integrates seamlessly with the AFM operation software. Firstly, the pressure is directly controlled from the Studio interface where it can be synchronized with the spectroscopy. Secondly, ViewPort allows targeting of cells or areas of interest directly in live feed of the optical camera and running a queue of positions. Furthermore, with CleanDrive, DriveAFM uniquely provides the PicoBalance functionality to measure the mass of microparticles or single cells by FluidFM using a frequency sweep or PLL.

Download the FluidFM brochure for more information
PicoBalance
PicoBalance: The unparalleled precision balance
Add-on for fast and accurate mass measurements of single cells or colloids
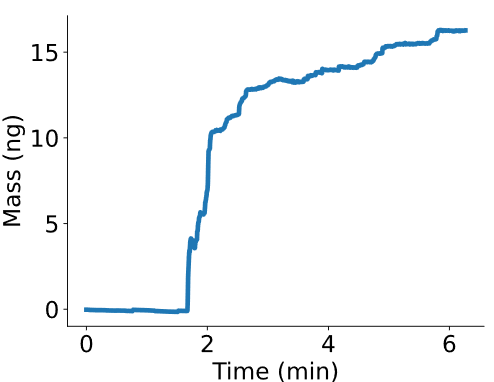
All over natural sciences and engineering mass is being measured to characterize and design a large variety of high performance systems. Yet there existed an instrumentational void for weighing microscopically sized systems such as single cells or colloidal microparticles. Motivated by this problem and driven by innovation, Nanosurf now offers a unique solution that allows nanotechnological measurements of a new dimension: the PicoBalance. Whether as a stand-alone instrument or as an add-on to the DriveAFM, the PicoBalance enables users to noninvasively measure microscopic biological or material science systems reaching sub-picogram and millisecond resolution.
Together with Prof. Daniel J. Müller of the ETH Zürich and Dr. David Martínez-Martín of the University of Sydney, Nanosurf has been developing the PicoBalance. The PicoBalance is able to measure the mass of even single adherent cells with 0.1% mass and millisecond temporal resolution.
In brief: a microscopic particle, like a cell, is attached to the PicoBalance's cantilever, which in turn is oscillated with very low amplitudes via photothermal-excitation. The mass of the attachment can be derived with high accuracy, and can be monitored over long periods of time, for biological samples even under physiological conditions.
Key Features & Benefits
- Direct measurement of the total mass in liquids
- Mass resolution: 5 pg; range: tens of pg to tens of ng*
- Time resolution: 100 ms; range: from seconds to days*
- Long-term stability enabling day-long experiments
- Compatible with LiveCell chamber, inverted light microscopy, FluidFM and DriveAFM * as measured with an NSC35
An integrated solution that combines mass monitoring with optical microscopy and long-term cell cultivation
The PicoBalance is the only research tool currently available that allows the recording of time-lapse mass measurements in physiological conditions, while simultaneously conducting fluorescence and/or differential interference contrast microscopy (DIC) in order to link cell mass dynamics to cell morphology and state.
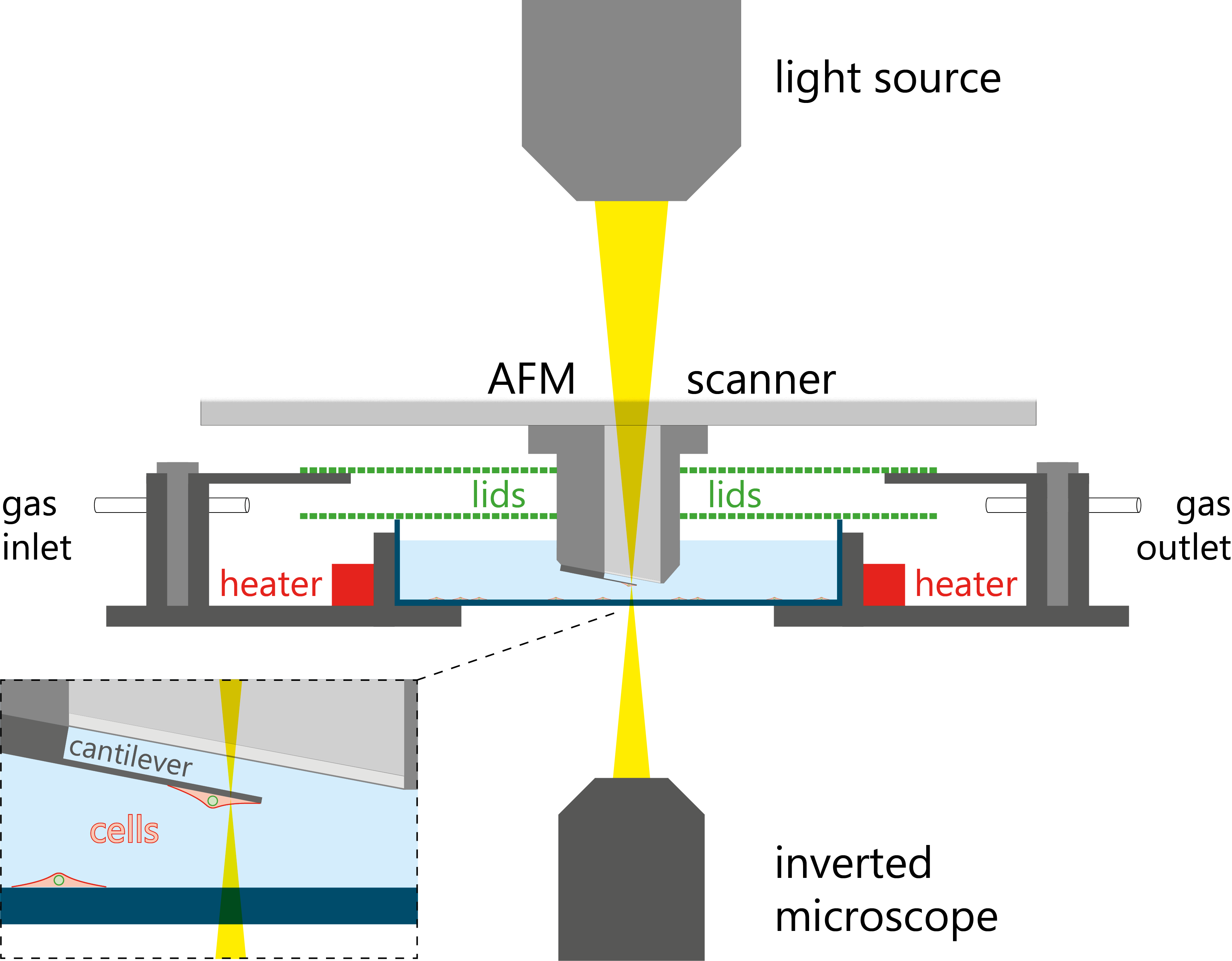
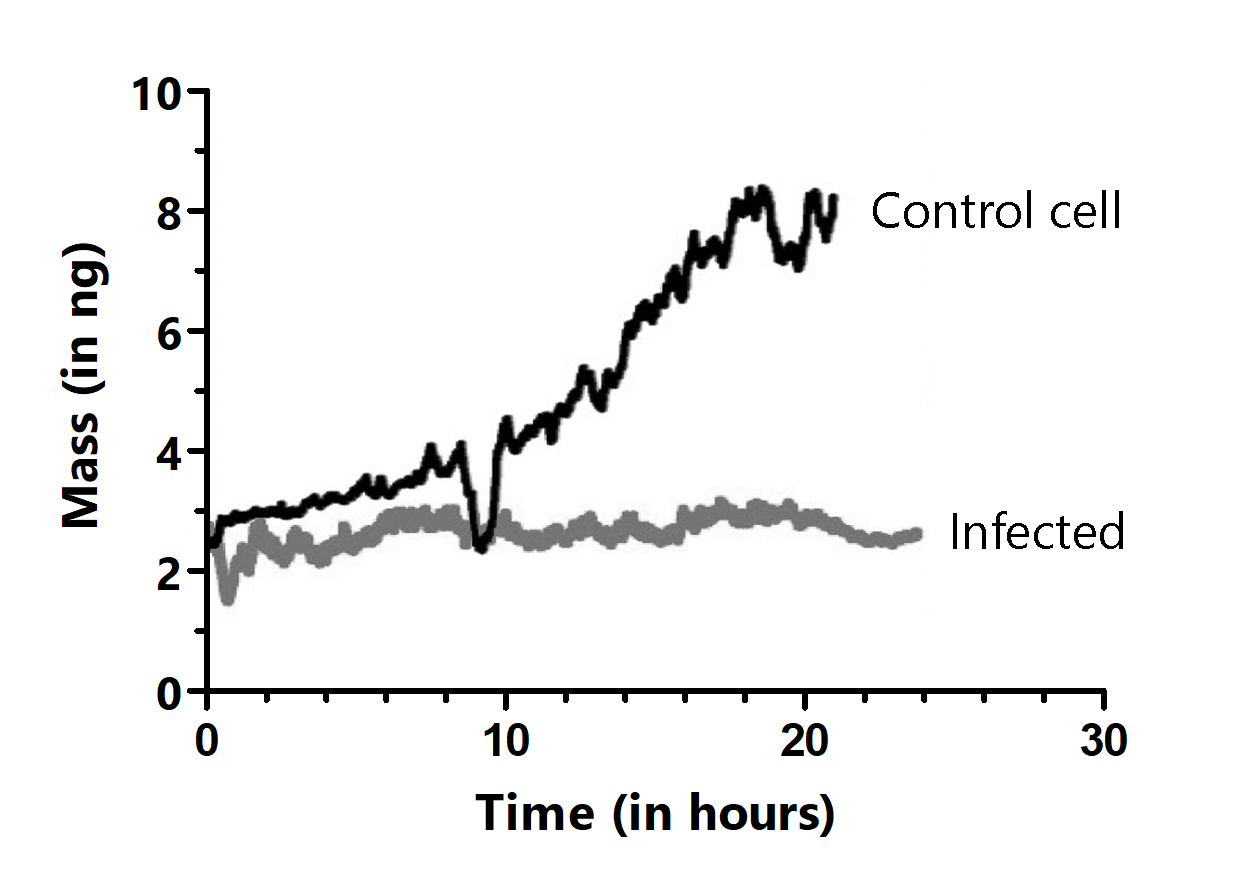
For further experimental details and results, please click here.
The PicoBalance allows novel research in the fields of cell mass or volume regulation, cell migration, cell nutrition, cell division and cell cycle progression, fat cell storage and metabolism, viral infection-related processes, ion channel properties, drug screening for drugs targeting pathways linked to cell growth, new therapies for cancer, aging, obesity, type 2 diabetes, neurodegeneration, and other diseases linked to a deregulation of growth control.
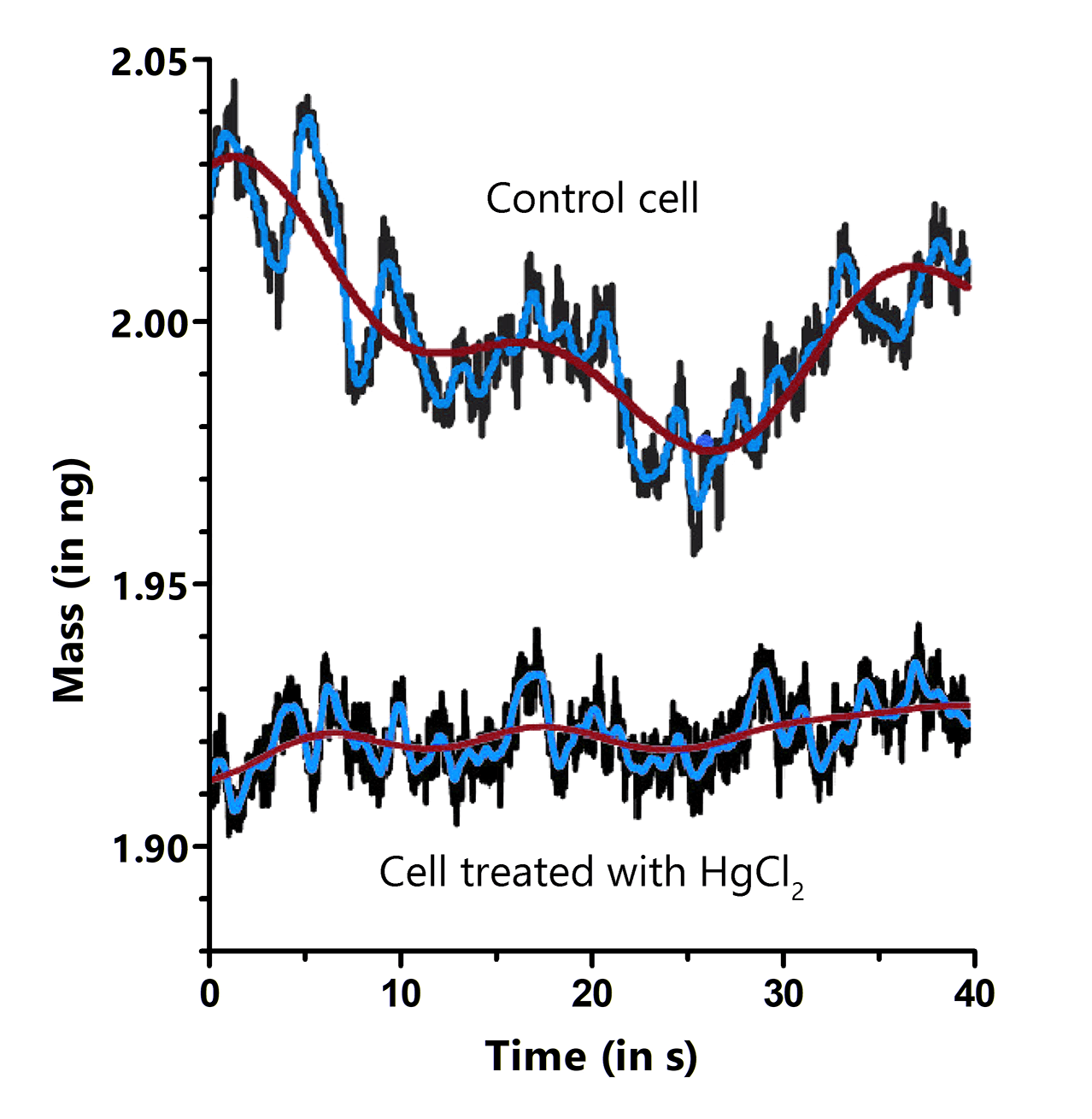
Groundbreaking research
In their bedrock Nature publication, Martínez-Martín et al. observed that the mass of living mammalian cells intrinsically fluctuates by around 1–4% over timescales of seconds throughout the cell cycle. Adding drugs to the medium, blocking water channels or the energy production of the cell, led to a vanishing of these fluctuations, showcasing. the power of the PicoBalance to study the effect of additives or other pharmaceutical components on cell mass development. It was also shown that growth and cell cycle progression are arrested in cells infected with vaccinia virus, but that mass fluctuations continue until cell death.
In the most recent Nature Communications publication , Cuny et al. stepped up the mass resolution an characterized the growth of yeast cells, which are up to 10 times lighter than mammalian cells. They found an unexpected growth behaviour: instead of growing linearly or exponentially in mass, they show several segments of growth before dividing, which challenges ideas of the growth regulation of these industrially and scientifically relevant organisms.
Interested in becoming a PicoBalance user yourself? Then contact us now to obtain access to this unique technology, which provides new insights cell biology and material science.
Characterizing material science systems
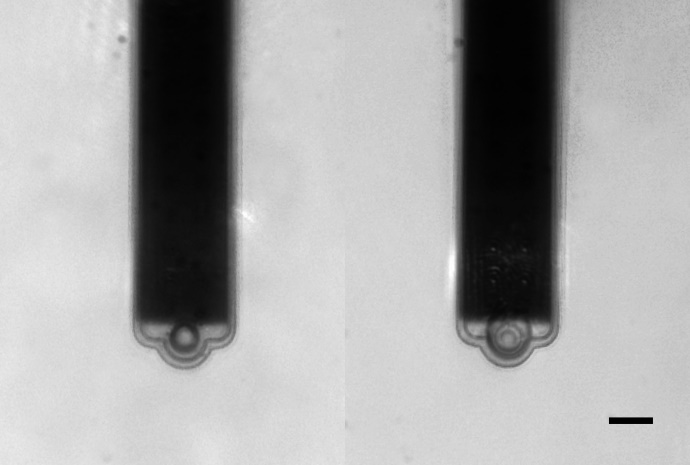
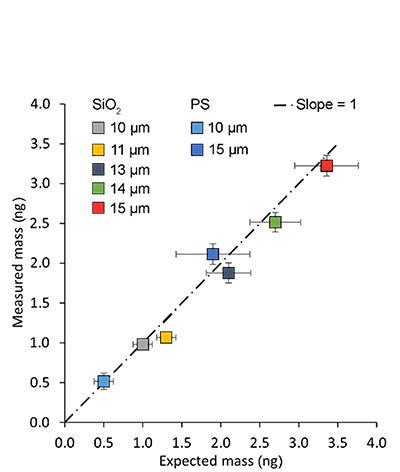
Mass determination with the PicoBalance is based on the measurement of the frequency shift that is induced by attaching the sample to the mass sensor, the cantilever. One area of application is the static size and mass characterization of colloidal systems, and their dynamic swelling properties in solvents. The combination with the FluidFM technology is of particular advantage – it allows fast and reversible attachment of the systems or the research of droplet-liquid interactions. Nanosurf has refined the mass measurement workflow and supplemented it with automatic procedures for preparing and executing the measurement.
Getting a new perspective on cellular systems
The PicoBalance also reveals new perspective on cellular systems found in fundamental, biotechnological and pharmacological research. Cells of varying sizes can be hosted on the cantilever, made to adhere by physiologically relevant substrates such as collagen, fibronectin, laminin etc. Researching the cell growth behaviour or the influence of chemicals on the cellular proliferation or function. The PicoBalance unlocks new applications in cell nutrition, mass regulation, disease related processes, and drug screening. The compatibility with transmitted light and fluorescence microscopy allows the correlation of mass measurements with cell morphology or cell state. All measurements can be performed under physiological conditions.
Image width: 110 µm
At the forefront of science
Interested in becoming a PicoBalance pioneer yourself? Then contact us now to be among the first researchers to obtain access to this unique instrument, which provides new insights into the most fundamental processes in cell biology.
Glovebox
DriveAFM glovebox workstation
Atomic force microscopy is uniquely suited to measure samples in-situ to monitor real-time, nanoscale surface phenomena. Research in energy storage, catalysis, 2D materials, semiconductors (ALD/MBE), organometallics, photovoltaics, and electrochemistry can often be air-sensitive and require demanding inert gas environments afforded by a glovebox. However, placing an AFM in a glovebox brings challenges such as limited sample access, cumbersome instrument operation, not to mention the additional sources of noise generated by the glovebox that can affect instrument’s performance.
Nanosurf has you covered: our turn-key glovebox AFM solutions offer a low-noise, high-fidelity nanoscale platform coupled with the most stringently controlled environment provided by the glovebox, while also addressing access and ease of use.
Nanosurf has integrated two different AFMs into a high-performance glovebox by collaborating with Jacomex, a leading manufacturer of gloveboxes. This turn-key glovebox solution means that you don’t have to worry about cutting corners on your AFM measurements to keep your high-purity environment for your experiments.
AFM glovebox workstation package overview
- <0.1ppm O2 and H2O environments for sample preparation and measurements
- Integrated signal feedthrough to enable AFM modes
- Reinforced table & frame to support the AFM and vibration isolation mass
- Vibration dampening to ensure optimal AFM performance
- Large load locks and touch controls to maintain environmental conditions
- Regeneratable oxygen catalyst
- Ease to access, magnetic cantilever holders which are easy to remove and kinematically align upon replacement
- Self-aligned or motorized cantilevers)
- Fully motorized AFM approach and engage
- Motorized sample stages
- Clear, crisp top-down optics for sample alignment (capable of <2µm lateral resolution)
What makes a good AFM for glovebox measurements?
The design and configuration of the AFM is tremendously important to be successful when operating in a glovebox. There is limited space within the glovebox, so a small footprint is desirable. Working with the gloves can be cumbersome, especially manipulating small objects like AFM cantilevers or needing to make multiple, manual adjustments to the AFM to initiate a measurement. Therefore, minimal manual manipulation is ideal to perform AFM for routine measurements. Samples can come in a variety of sizes or substrates, so an AFM capable of accommodating a wide variety of samples without additional preparation simplifies operation of the system.
What makes a good glovebox solution for performing AFM measurements?
An ideal AFM environment is diametrically opposite to that found within a glovebox. Gloveboxes maintain an inert gas environment for work with contamination-sensitive or air-sensitive materials. By design, they precisely control the atmosphere and prioritize design features such as temperature stability, O2 permeability, water content, airlock integrity, and pump performance. The glovebox with all the pumps for atmospheric conditioning introduce vibration, acoustics, and airflow which adversely affect the performance of the AFM. In contrast, an ideal AFM environment is free of excess vibration, has limited airflow, is temperature stable and acoustically quiet over a period of hours. Therefore, careful consideration about instrument compatibility and requirements are needed and not all AFMs are created equal for glovebox work.
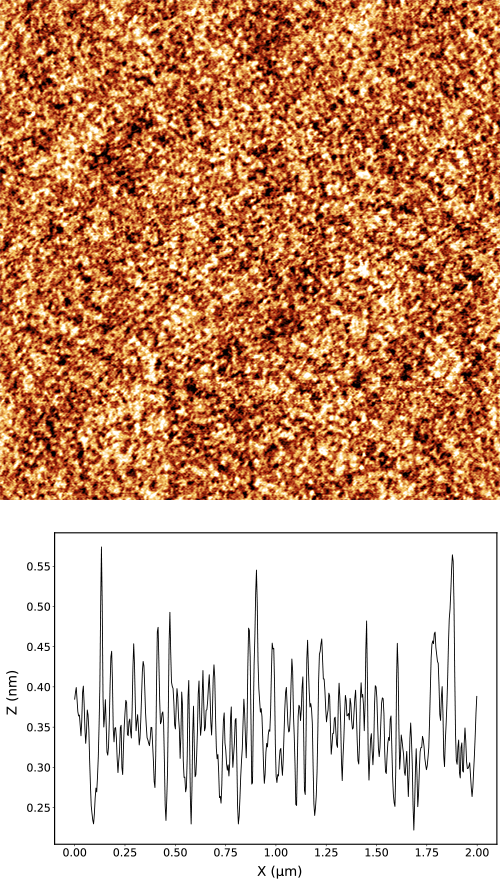
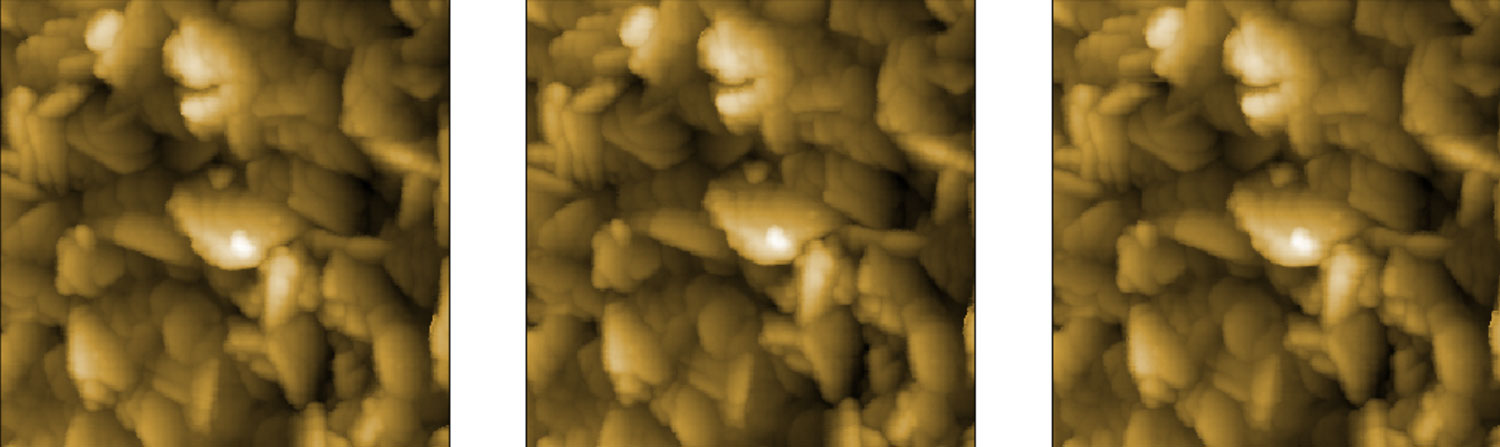
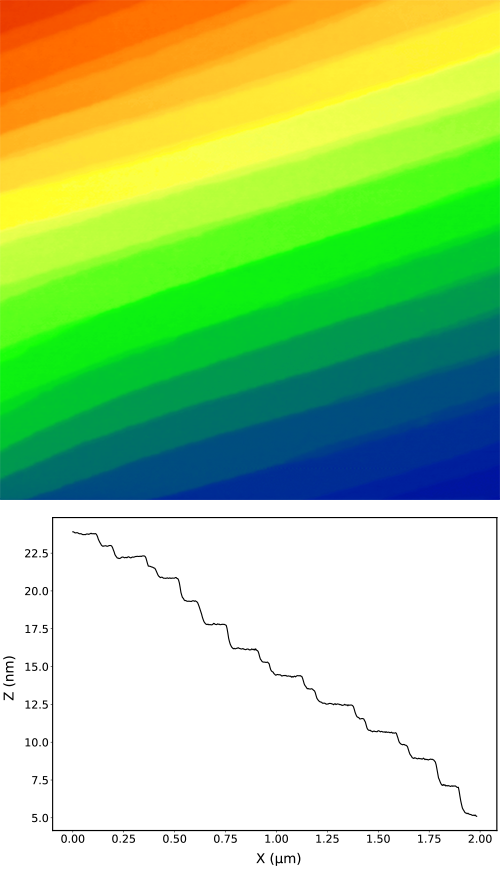
Polished silicon wafer
Left: A polished silicon wafer has typically very small roughness. In our case the roughness of 80 pm was measured with the noise floor of 20 pm. The image of the wafer shows easily recognizable granular structure and an excellent resolution of details. The cross-section of the image demonstrates that the data is dominated by the physical roughness of the sample and the vibration noise is not noticeable.
Silicon carbide crystal
Right: a polished crystal of silicon carbide (SiC) shows clear atomic terraces with spacing of 1.5 nm and the intermediate terraces with spacing of 0.75 nm. This excellent height resolution is what is typically required for the study of graphene and other 2D materials.
Both samples were measured with a DriveAFM in a Jacomex glovebox.

Nanosurf's glovebox AFMs...
- ...use a tip-scanner design to ensure the samples are easy to access to load/retrieve regardless of size/shape of substrate (up to 100 mm diameter)
- ...have a compact footprint that saves glovebox real estate.
- ...use a motorized sample engagement and stage translation to allow for the operator to run the AFM without excess ‘hand-in-glove’ time. Once the sample is on the AFM, the entire system is controlled via software from outside the glovebox.
- ...having automated laser/photodetector alignment (DriveAFM) allows for hands-free setup of the system for different cantilever shapes/sizes needed for advanced AFM characterization.
Whether you are looking for a glovebox tool to simply characterize materials in process or your aim is to bring a top-class nanoscale research station into a glovebox environment, Nanosurf’s turn-key glovebox AFM solutions have you covered. The DriveAFM is the premier research AFM platform for research that can’t afford compromise.
— Dr. Denis Vasyukov, Product Manager Glovebox AFM Solutions, Nanosurf AG
- The inherent compact design saves floorspace without compromising on performance and gives more room in the glovebox for sample preparation and other experiments.
- Samples stages and approach/engagement are motorized, meaning once the sample is loaded on the stage, all control of the AFM happens via software. Nanosurf AFMs are tip scanners which allow for a flexibility for the size/shape of the samples to be measured.
- The DriveAFM has additional features that make it easier to use, and provides higher performance in a glovebox. DriveAFM has a fully motorized optical path allowing alignment of the laser and detector can be controlled via the software.
- There are no small, cumbersome, delicate knobs to turn to load a sample or align the AFM for operation.
What are the applications for a Glovebox AFM?
Any application which requires environmental control, especially of O2 and H2O contamination, will require putting the AFM in a glovebox. Such applications include 2D materials research, semiconductor nanoscale analysis, Lithium (or other Alkali metal) research for energy storage, ALD/MBE materials research, free radical surface work, molecular machines and more.
The DriveAFM is the solution of choice for applications where a detailed analysis is necessary, such as measuring the magic angle of bilayer 2D materials via nanoelectrical characterization. The standard DriveAFM offers performance without compromise, bringing together world-class AFM performance with the flexibility of a large scanner and faster acquisition speeds. The DriveAFM's glovebox integration brings this market leading system into the glovebox.
Accessories
DriveAFM accessories

Standard sample holder
Samples up to 100 mm in diameter, central magnet to hold small samples on pucks, clips to hold larger samples.

Petri dish holder
Holds petri dishes of 35 or 50 mm diameter and height up to 10 mm.

Heater sample holder
Heats samples up to 250 °C. Needs TEC controller CH1.

Cooling & heating sample holder
Temperature range of -35 °C to 180 °C. Needs TEC controller CH1, pump and temperature reference unit.

TEC controller CH1
Temperature monitoring and control, temperature stability <0.05 °C.

Pump and temperature control unit
Used with cooling & heating sample holder. Includes a pump and temperature control unit for the circulating coolant.

C-AFM sample holder
Sample holder for advanced conductive AFM option. Current range of ± 25 nA with a sensitivity of 108 V/A. Current noise 1.5 pA at 2 kHz bandwidth.

150 µm z-stage
Z-range extension of up to 150 µm for long range force spectroscopy experiments. For petri dishes of 35 mm diameter and 50 mm diameter.

Variable magnetic field generator
Variable in-plane magnetic field, integrated Hall sensor, Max. field – 720 mT (2 mm gap), field resolution – 0.1 mT, Sample size - up to 10 x 10 mm2.

Digital inverted microscope option
The DIMO can be integrated with Isostage 300, SMA optical port for illumination, Motorized centering and focus, Nikon objective, Fluorescence option with a fiber coupled light source.

EC cell
For electrochemical experiments, PEEK or PVDF EC cell in a stainless-steel frame. Built-in electrode and tubing feedthroughs.

Environmental control chamber
For sample measurements under controlled environments. Expanding sealing membrane. Gas inlet and outlet.

DriveAFM signal I/O module
Allows direct access and control of many AFM signals by external hardware.

DriveAFM tip current module
3-stage current amplifier for small currents allows precise measurements of local sample conductivity in C-AFM mode, and use of tunneling current as Z feedback in STM mode. Both tip bias and sample bias are possible.
- Current ranges:
±0.5 mA, ±5 μA, and ±50 nA - Current noise:
1.3 pA/√Hz, 0.13 pA/√Hz, and 0.01 pA/√Hz
Acoustic isolation

AE 350
Acoustic enclosure for the stand-alone setup

Acoustic enclosure 1100
- Protect your equipment from airborne noise emitted by air conditioning, venting, door slamming etc.
- Enables you to perform undisturbed experiments with your inverted optical microscope setup.
Software
Nanosurf Studio for DriveAFM
Nanosurf continues to deliver. DriveAFM, the revolutionary AFM system, demonstrates what engineering is capable of today. Now, with Nanosurf Studio, we are handing you the software suite to round off the superior Nanosurf experience. The new control software for DriveAFM is an intuitive platform made for performing your AFM measurements efficiently and easily.
Our software engineers and application scientist took a good look at what performing atomic force microscopy measurements is actually like from a user perspective, and went back to the drawing board to create a user experience that allows you to focus on your measurement and your results. Look forward to a new release each quarter, with new features and improvements. Just press play.
Nanosurf Studio: the future-proof AFM software platform
Set up your individual software GUI – for experts and novice users
Automated AFM setup (laser alignments, spring constant and sensitivity calibration, approach, data acquisition)
Viewport optical sample navigation
Quarterly releases with new features, improved workflows and bugfixes
Easy to learn for novice users, all the flexibility for the expert user
Instrument control is fully scriptable
AutoAlign
AutoAlign boosts your experience and makes your life easier. With auto laser alignment you don’t have to worry about placing the laser on the cantilever. With the click of one button, the whole optical system will be aligned for you, the detector centered, and the spring constant and deflection sensitivity determined. Lean back, and just press play!
Viewport hybrid sample navigator allows you to optically navigate your sample with already scanned areas mapped over image
See in this clip how a square around the area of interest is selected on the live image from the optical microscope, adjusted, and followed by the AFM measurement.
Request Nanosurf Studio
Free lifetime updates: download all software updates for free
All software updates for Nanosurf Studio and legacy control software are free of charge. Our software team is constantly working on new features and improvements to make the user experience better, more intuitive and more efficient.
Legacy control software
Automatic/parameter-free frequency tuning based on cantilever characteristics
Simply choose the cantilever you are using, and the system automatically performs the frequency sweep prior to approaching the sample. No manual setting of parameters is required.
Distance measuring tools: measure the distance between points or lines, the height of features, and more
A selection of different measuring tools allow you to accurately measure angles and distances directly on the acquired measurement image.
Determine the distance between two points or between two parallel lines to make very precise measurement (as shown in the video).

Scripting interface: Python package for COM interface
Python API for data acquisition and control of Nanosurf atomic force microscopes
At Nanosurf we realize that leading researchers are often interested in modifying the standard routines of an instrument, or else everyone would be doing the same thing with the same hardware.
Sometimes there are also application-specific routines, the scope of which we cannot predict and program ourselves. This is why we developed the Nanosurf Scripting Interface.
This interface can be used for automation of routine tasks and for creating new experiments. It gives the users full control over our user interface, and some control over the hardware functionality.
The Nanosurf control software publishes its functionality via the COM interface, by running an instance of COM Automation Server. COM Automation Clients can ask the server during the runtime about its functions and access them. These functions can be accessed through most modern programming languages: Python, C++, C#, VBS, Matlab, JS, LabView, etc.
For ease of use, Nanosurf has created a Python API for its COM interface. We chose Python as our API language, because of its ease of use and learning, popularity and universality, and because of number of data and image processing libraries used in academia and industry.
The Nanosurf Python package can be downloaded and installed from PyPI using pip:
pip install nanosurf
Nanosurf Python Package
See how to take control over the GUI settings in Python. The video shows the most basic actions, like changing the imaging mode, choosing the right cantilever, adjusting image parameters and the PID settings. See how to automatically find the working frequency, start the approach, start scanning, and save the data.
On-demand webinar on Nanosurf's Python API
In this webinar we introduce the Nanosurf Python API and learn how to control Nanosurf instruments with it. We also briefly touch on the processing of the data, stored in NID files, and look at how to create custom user interfaces.
Additional usability features
- Spectroscopy wizard: follow easy steps to set up spectroscopy measurements
- One software UI for all scan heads: no additional learning curve if you use multiple Nanosurf AFM systems
- Automated deflection calibration
- UI layouts for beginners and advanced users
- Highly configurable graph area with mode-dependent auto-layout: the software automatically shows the relevant graphs and information
- Easy file handling with comfort features: auto apply naming conventions, Windows Explorer integration, image gallery, bulk renaming.
Related content
Application notes, technical notes, publications
Reconstituted virus–nucleus system reveals mechanics of herpesvirus genome uncoating
Abstract The viral replication cycle is controlled by information transduced through both molecular and mechanical interactions. Viral infection mechanics remains largely unexplored, however, due...
Read moreStudies of Self-Organization of Semi-Fluorinated Alkanes on Different Substrates with DriveAFM
The capabilities of visualization of semi-fluorinated alkanes with dynamic mode AFM are demonstrated in this document. Diverse nanoscale structures were observed in adsorbates of semi-fluorinated a...
Read moreInjection into and extraction from single fungal cells
The direct delivery of molecules and the sampling of endogenous compounds into and from living cells provide powerful means to modulate and study cellular functions. Intracellular injection and ext...
Read moreMitochondria transplantation between living cells
Mitochondria and the complex endomembrane system are hallmarks of eukaryotic cells. To date, it has been difficult to manipulate organelle structures within single live cells. We developed a FluidF...
Read moreMagnetic Surfaces for Photo-Isomerization of Azobenzene Based Polymer Probed Using Magneto Optica...
We have studied the intervention of catalytically active nickel surface on trans–cis isomerization of azobenzene moieties, specifically in the novel polyethylene glycol itaconate-azobenzene based s...
Read moreBreakthrough instruments and products: DriveAFM for high-performance atomic force microscopy
Atomic force microscopy is a powerful technique for measurement and mapping of nanoscale topography and electrical and mechanical sample properties. The Nanosurf DriveAFM is a new generation instru...
Read moreExploring Nanoscale Organization of Normal Alkanes on HOPG Substrate with DriveAFM
AFM studies of normal and ultra-long alkanes reveal a set of fine structural features related to the lateral chain folding and lamellar architecture in their layers on HOPG. The formation of these...
Read moreCharacterization of Polyethylene with DriveAFM
Polyethylene (PE) is a semicrystalline polymer and is one of the most widely produced plastics globally for use in consumer goods and packaging with wide-ranging applications. Shopping bags, milk b...
Read moreEvaluating AFM as an analysis tool for graphene
Graphene is the most famous member of the 2D materials family: a sheet of covalently bonded carbon atoms in a hexagonal lattice in which the thickness has been reduced to a single atom. This unique...
Read moreDetermining mass of individual micron-sized particles using PicoBalance and FluidFM® probes
Micron-sized particles are commercially available in a wide range of materials such as polymers, (magnetic) metals, glass and ceramics. They have applications in biology, medicine, material science...
Read moreVariable magnetic field generator
The variable magnetic field generator is an accessory for Nanosurf’s CoreAFM, FlexAFM and DriveAFM. The VMFSH enables AFM measurements in a variable in-plane magnetic field.
Read morePiezo-response Force Microscopy (PFM)
Piezo Response Force Microscopy (PFM) The word “piezo” is derived from the Greek word piezein, meaning “to press tightly”. Piezoelectricity is the ability of a material to convert mechanical...
Read moreCleanDrive: Photothermal Excitation of the Cantilever
Nanosurf introduces CleanDrive - a clean and reliable way to oscillate an AFM cantilever using photothermal excitation. CleanDrive uses a near IR 785 nm laser aimed at the base of the cantilever to...
Read moreWebinar: Piezo Force Microscopy (PFM)
Piezo Force Microscopy (PFM) Speaker: Dr. Patrick Frederix In this webinar, Dr. Patrick Frederix presents Piezo Force Microscopy and describe its implementation on Nanosurf instruments. D...
Read moreWebinar: PicoBalance — Mass measurements with DriveAFM
PicoBalance: Mass measurements with DriveAFM Speaker: Gotthold Fläschner In this webinar, Gotthold Fläschner presents the concept of PicoBalance and explains its working principles. Examp...
Read moreWebinar: Advanced KPFM techniques — Comparison between AM KPFM and FM KPFM
Advanced KPFM techniques: Comparison between AM KPFM and FM KPFM Speaker: Dr. Denis Vasyukov KPFM is an essential technique for nanoelectrical characterization of materials and devices. W...
Read moreWebinar: Introducing the DriveAFM high-end atomic force microscope system
Introducing the DriveAFM high-end atomic force microscope system Speaker: Dr. Christian Bippes In this webinar Dr. Christian Bippes explains the technology behind the DriveAFM, as well...
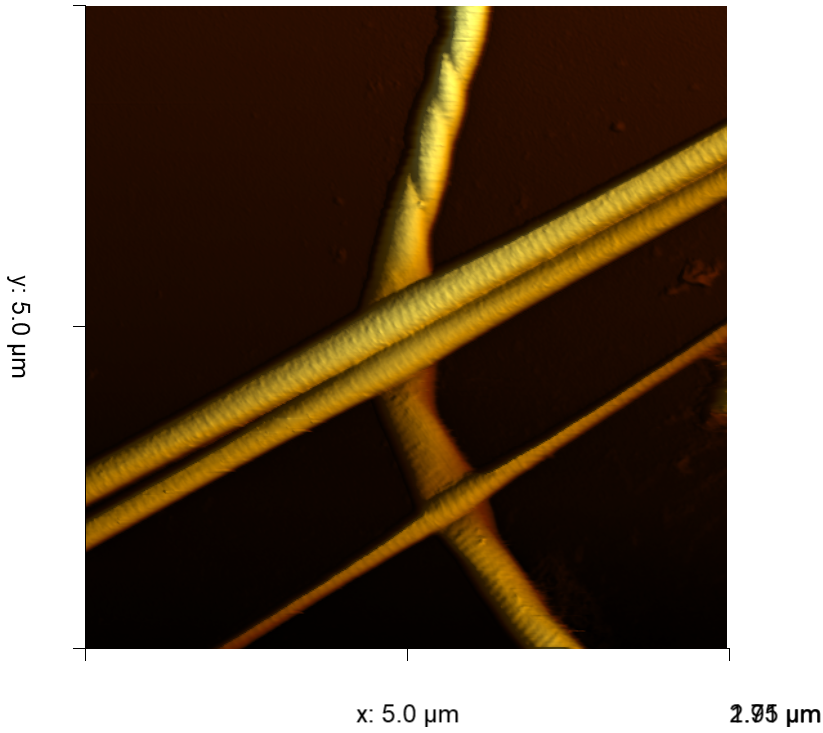
Read morePlacing a cantilever on the DriveAFM cantilever holder
Tutorial: Placing a cantilever on the DriveAFM cantilever holder See how to place a cantilever on the cantilever holder for the Nanosurf DriveAFM.
Read more