J. Chem. Educ., 2018, 95 (2), pp 315–319
Wesley C. Sanders*, Peter Iles, Ron Valcarce, Kyle Salisbury, Glen Johnson, Aubry Lines, John Meyers, Cristofer Page, Myles Vanweerd, and Davies Young
This paper describes a laboratory exercise used to address the ongoing need for nanotechnology-related, hands-on laboratory experiences for undergraduate students at Salt Lake community college. Students used cyclic voltammetry to analyze silver nanogrids printed using microcontact printing and subsequent metallization. The silver nanogrids exhibit electrochemical behavior similar to that of electrodes manufactured in industry. The experiment provided students with an opportunity to create conductive silver nanogrids using polymeric templates, and to gain experience using instruments such as a sputter coater, an SEM and an AFM. Optical microscopy, atomic force microscopy, and scanning electron microscopy were used to assess nanogrid quality and dimensions.
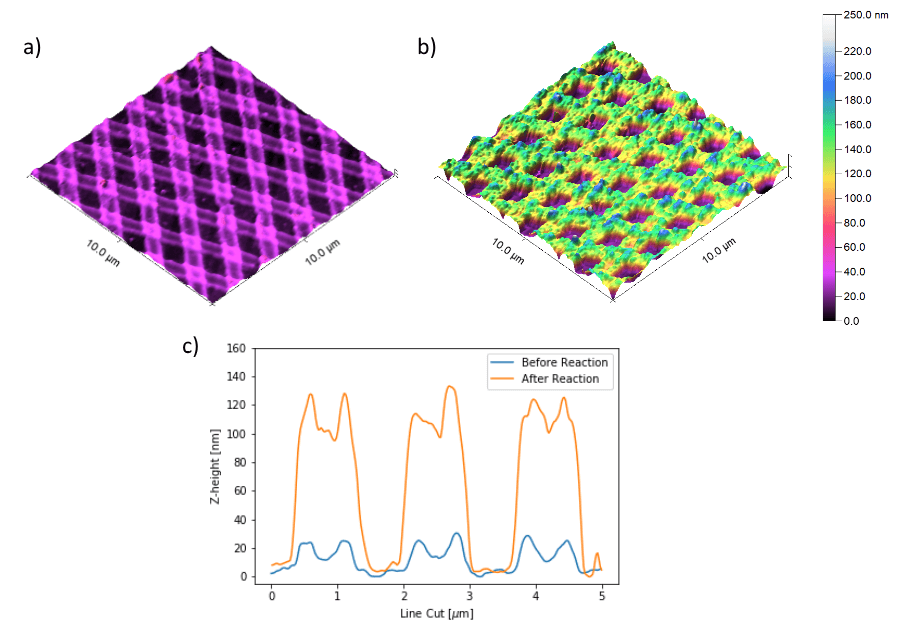
For the AFM analysis a Nanosurf Easyscan 2 AFM (the predecessor of the NaioAFM) was used to measure the structure height increases that amounted to over 100 nm, attributed to the formation of silver metal on the template.
For measurements in liquid, we recommend CoreAFM.

