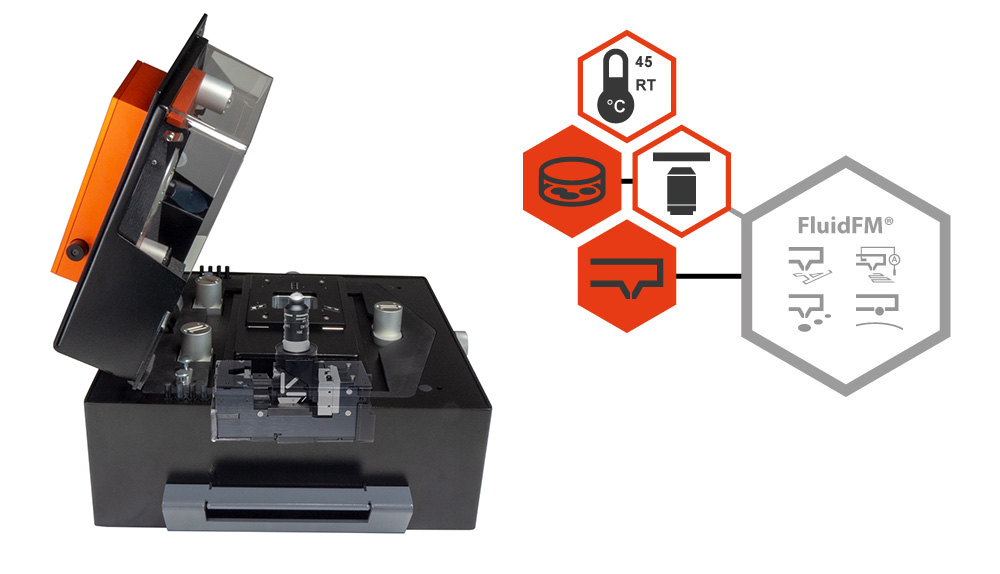
Integrated inverted microscope functionality for CoreAFM
| View samples from below | |
| Exchangeable objective lens | |
| Enhances FluidFM® functionality |
The CoreAFM is a very versatile atomic force microscope that can be used for a multitude of different types of research. The digital inverted microscope option (DIMO) enhances your CoreAFM to allow optical view from below, replacing the need for a completely different AFM setup with an inverted microscope.
Enhanced viewing ability
The DIMO empowers you to conduct advanced experiments that require an inverted microscope, as is the case for many Bio AFM applications. It enhances your capabilities when using accessories like the petri dish holder (visible at the beginning of the video to the right), and does not obstruct any standard accessories that fit your CoreAFM (with the exception of the variable magnetic field generator).
Fluorescence measurements
The DIMO uses the internal illumination source for bright-field transmission optical microscopy. With the fluorescence option, light from an optical fiber can be easily guided into the CoreAFM and DriveAFM for epi-illumination from the bottom.
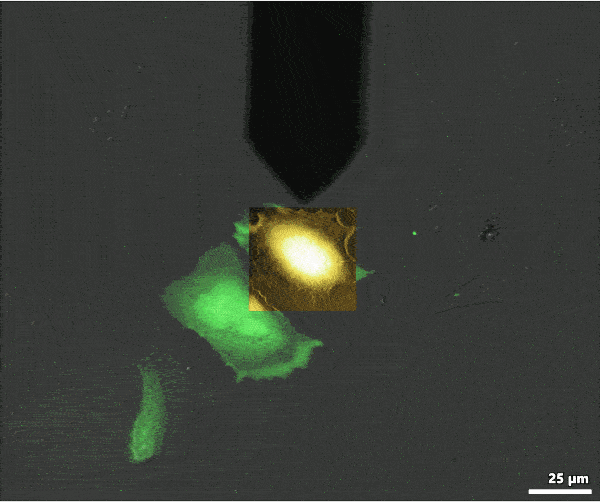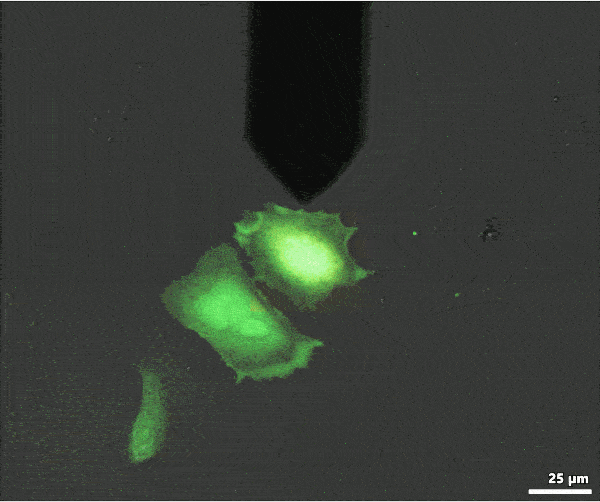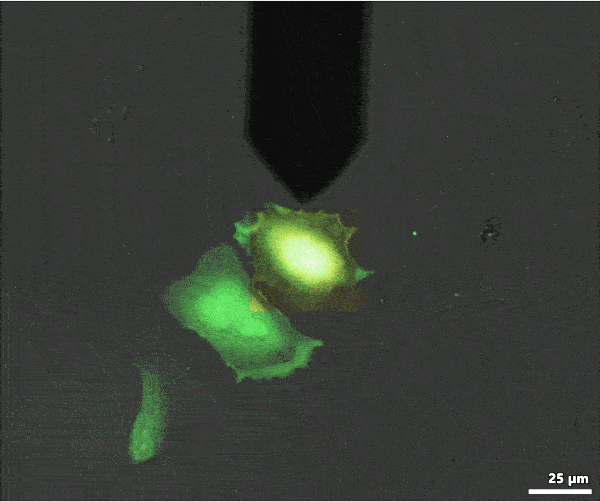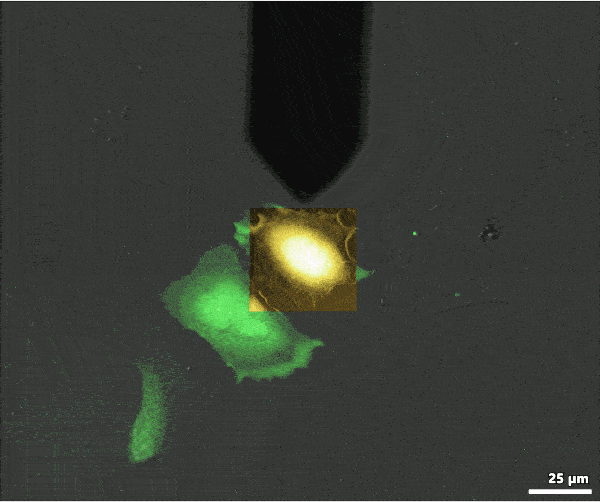
AFM data can be overlaid with the optical data to colocalize information from each imaging mode. The image shows an overlay of AFM topography with fluorescence (green) and transmission light (gray) optical microscopy images. The overlay was created with MountainsMap image processing software.
Even better with FluidFM® functionality
Nanosurf has a long-standing collaboration with Cytosurge and has been developing FluidFM® solutions for FlexAFM since 2011, offering powerful applications for single-cell analysis and manipulation.
The DIMO upgrades the CoreAFM to be capable of performing FluidFM® on biological samples too.
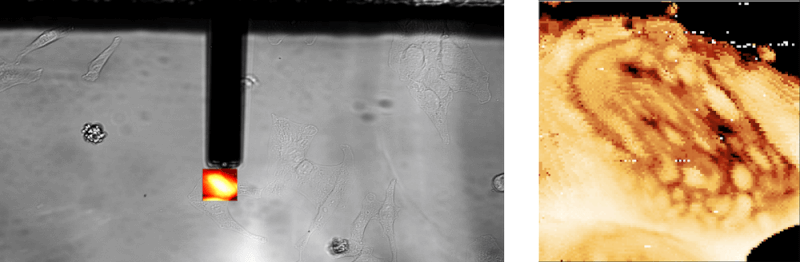
Below is an example of FluidFM® colloidal spectroscopy on HeLa cells: the powerful digital microscope allows you to easily view cells, maneuver the cantilever, and carry out your single-cell manipulations.
Motorized function for cantilever centering and focus
FluidFM® spotting
Single cell injection with FluidFM
High-powered microscope optics
- Objective: 20× 0.4 NA long working distance objective from Nikon
- Objective is interchangeable between experiments with other Nikon objectives that have a M25×0.75 thread and 60-mm parfocal length
- Camera: Highly sensitive 2.3 MP monochrome CMOS camera with 5.86 µm × 5.86 μm pixel size (eq. 1/1.2”), USB 3.0, frame rate up to 48 frames/s at full size
- Fluorescence option with fiber coupling and filter set that can be changed between experiments
- Filters are in a Nikon filter block, as used for Eclipse Ti series microscopes
- Optical resolution: 0.8 µm with default objective and transmission
- Motorized focusing and cantilever centering










