Isolated purple membranes reveal the trimeric organization of this bacterial transmembrane protein
Bacteriorhodopsin (BR) is a trimeric integral membrane protein found in the cell membrane of halobacteria. It acts as a proton pump that captures light energy and uses this to move protons across the membrane. The thus created proton (or pH) gradient is subsequently used to generate ATP, the chemical energy source of many processes inside a cell.
Bacteriorhodopsin is often found in 2-dimensional crystalline patches known as "purple membrane", which were studied here.
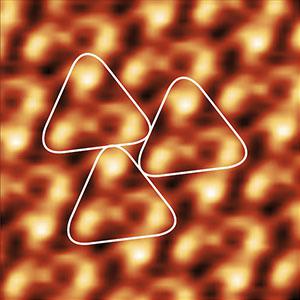
The cross-correlation average in picture 1 was calculated from the overview image in picture 3 using the IPLT software. This software can be downloaded for free at www.iplt.org
Three bacteriorhodopsin trimers have been highlighted (white rounded triangles) for clarity.
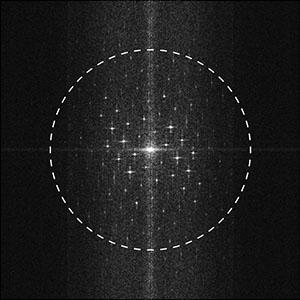
For 2-dimensional crystals, the resolution limit of recorded data can be estimated from a 2D power spectrum. In picture 2, diffraction spots go well beyond 1 nm lateral resolution (white circle).
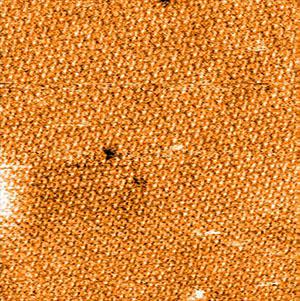
Picture 3 shows an overview image of the cytoplasmic side of the bacteriorhodopsin containing crystalline purple membrane. It was recorded in static mode using a FlexAFM scan head (10-µm; version 3) in combination with the C3000 controller and a cantilever with a nominal spring constant of 0.1 N/m (Uniqprobe, qp-CONT, Nanosensors). The image shows unfiltered data with linear background correction.

