Cells are the building blocks of life. Some thrive best individually, suspended. Most of them are however adhering cells integrated in a larger 3D matrix, like tissue. These cells interact with neighboring cells in the same tissue or those at the interface to adjacent, different, tissue. This can be a natural interface as between tendons and bones, or in the case of implants or biofilms, an artificial one. The forces governing cell-substrate and cell-cell interactions are important for their structure and function, and their quantification is of interest to better understand the mechanical strength of tissue and interfaces and related failures therein. Single cell force spectroscopy measurements are a powerfull tool to this end.
Flex-FPM — The standard tool for single cell adhesion force spectroscopy
Atomic force microscopy (AFM) has been used extensively to study cell-substrate or cell-cell interactions at the single cell level [Helenius et al. (2008), Moreno-Encerrado et al. (2017)]. To perform single cell force spectroscopy cells are generally immobilized chemically to a cantilever. However, there are some drawbacks to this approach. The chemical immobilization limits the maximum obtainable adhesion forces to a few hundred nanonewtons. In addition it requires many cantilevers to obtain conclusive results. The limitation in force range is particularly troublesome when studying adhering cells after prolonged incubation times (hours to days), where forces may exceed the micronewton range. This is particularly true when studying confluent layers of cells. Here cell-cell interaction contributes in addition to the substrate adhesion that is generally studied.

Flex-FPM (Fig. 1) overcomes these two main limitations. Flex-FPM is a flexible tool combining the FlexAFM atomic force microscope and FluidFM. Cell adhesion measurements were pioneered at the ETH Zurich in the groups of Prof. Julia Vorholt and Dr. Tomaso Zambelli. Using FluidFM™ technology different cell types can be attached to the cantilever via negative pressure through a channel inside the cantilever. FluidFM has been demonstrated for measuring the cell substrate adhesion of many different cell types, like mammalian cells, yeast cell and bacterial cells. Compared to chemical binding, with FluidFM much higher forces can be exerted after only a few seconds contact time. The addressable detachement forces for mammalian cell substrate adhesion reached into the low micronewton range [Potthoff et al. (2012); Potthoff et al. (2014)]. Data sets on different surface textures revealed a strong dependence of the cell substrate adhesion with surface texture depth. These adhesion measurements may assist in a rational design of the surface texture to improve coverage of cardiovascular implants like stents with endothelial cells. This endothelialization is critically important to prevent blood-foreign body interaction. Insufficient endothelialization can lead to inflammatory reactions, acute thrombosis or chronic stenosis, i.e. the narrowing of the liminal cavity [Potthoff et al. (2014)]. Despite the high forces, the force resolution is still sufficient to measure the adhesion force of single membrane tethers from the extracellular matrix; this was shown for example for single cell force spectroscopy experiments to study bacterial adhesion forces[Potthoff et al. (2015)].
The binding via aspiration is not only strong and fast, but also reversible. Consequently, the same FluidFM™ probe can be used for multiple cells in a row. A magnificent number of over 200 different yeast cells was measured with a single cantilever. This was done in one day under varying environmental conditions [Potthoff et al. (2012)]. The selection of cells was easily done using the inverted optical microscope. The cells were selected based on the cells shape (morphology), cell size or specific protein presence using a fluorescence marker. Also for mammalian cells, the throughput is higher than with chemical immobilization. The interaction of the cell surface of mammalian cells to a cantilever is more complex than that of yeast cells. This causes more aspecific adhesion, requiring cleaning of the cantilever between experiments. Protocols have been established to clean the cantilever enzymatically with trypsin [Potthoff et al. (2014)] or chemically in a sodium hypochlorite solution [Jaatinen et al. (2016)]. After cleaning, new cells can be aspired without need for a new coating step as would be the case for conventional, chemical binding.
Cell-cell adhesion forces
Recently, FluidFM™ cell adhesion measurements were extended to study cell-cell interaction. This can be the force between a cell (on the cantilever) and a cell below on a substrate (fig. 2 A), but also between a cell and its surrounding cells in a confluent layer (fig. 2 B).
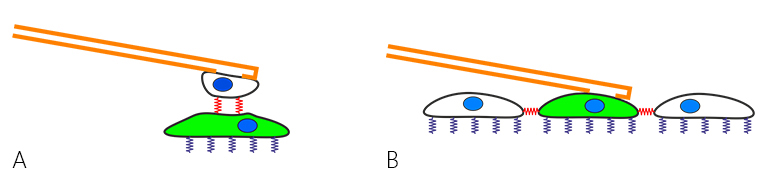
Dr. Noa Cohen of Prof. Tanya Konry's group at Northeastern university in Boston studied cell-cell adhesion forces with a Flex-FPM system to gain more insight into tumor progression and metastasis [Cohen et al. (2017)].
Figure 3 shows an optical image of the method depicted in fig. 2 A that was used by Cohen for this study.
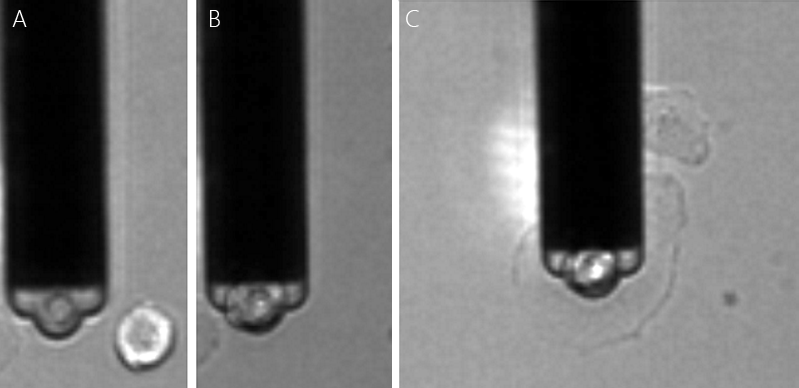
Single MCF7 breast cancer cell were immobilized on the cantilever. The cell was then pushed on different cell types that were immobilized on a substrate. The measured cell adhesion between MCF7 cancer cells to different cell types were found to develop differently with incubation time. In these experiments, the reversible binding of cells allowed the different cell pairs to be studied with the same probe (fig. 4), allowîng better comparison of measurements.

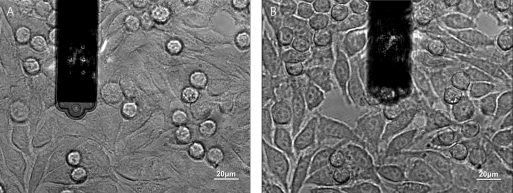
Dr. Ana Sancho from the group Prof. Jürgen Groll's group at the University of Würzburg extensively studied the interaction between a cell and its neighbors in a confluent layer of epithelial cells (fig. 2 B) [Sancho et al. (2017)]. Fig. 5 shows the cantilever picking up a cell from a confluent layer (A). After removal the empty space from where the cell was picked up is visible (B). Again, the epthelial cells of interest could be selected based on cell size and cell shape using the inverted microscope.
Human endothelial cells from the umbilical artery were found to exert strong intercellular forces (figures 6 A & B). The forces could be decreased significantly by overexpression of the so-called Muscle Segment Homeobox 1 (MSX1). The MSX1 induces a endothelial-to-mesenchymal transition. This transition is a process involved in cardiovascular development and disease. Complementary to these adhesion experiments, the Flex-FPM system was also used to perform nano-indentation measurements. To this end colloidal beads aspired to the cantilever and force curves were recorded on the cells.
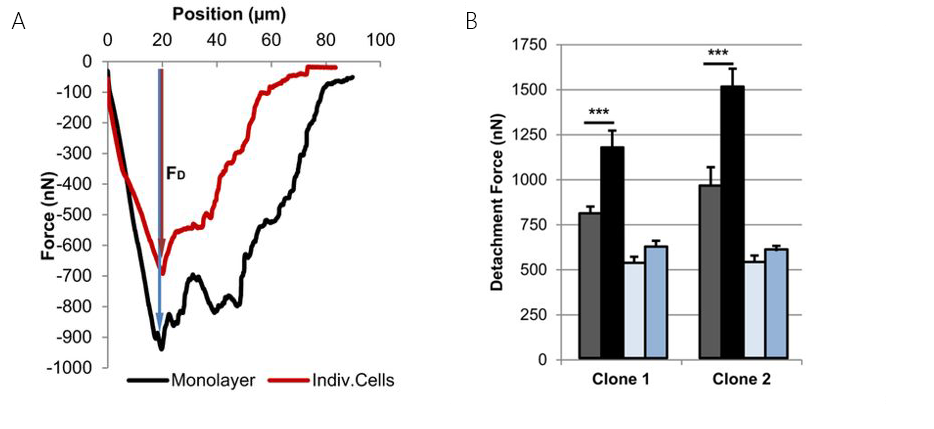
Both examples strongly benefitted from FluidFM™ technology provided by the Flex-FPM solution. In case of the confluent layer the large forces of up to over 1.5µN eliminate chemical binding to study cell-cell adhesion forces. In both cases the reversible binding provided the experiments with the necessary speed-up to obtain sufficient statistics.
References
Jonne Helenius, Carl-Philipp Heisenberg, Hermann E. Gaub, and Daniel J. Muller (2008). Single-cell force spectroscopy. Journal of Cell Science 121: 1785-1791; doi:10.1242/jcs.030999
Alberto Moreno‐Cencerrado, Jagoba Iturri, Ilaria Pecorari, Maria D.M. Vivanco, Orfeo Sbaizero, and José L. Toca‐Herrera (2017). Investigating cell‐substrate and cell–cell interactions by means of single‐cell‐probe force spectroscopy. Microscopy Research & Technique 80: 124-130; doi:10.1002/jemt.22706
Eva Potthoff, Orane Guillaume-Gentil, Dario Ossola, Jérôme Polesel-Maris, Salomé LeibundGut-Landmann, Tomaso Zambelli, and Julia A. Vorholt (2012). Rapid and Serial Quantification of Adhesion Forces of Yeast and Mammalian Cells. PLoS ONE 7: e52712; doi:10.1371/journal.pone.0052712
Eva Potthoff, Davide Franco, Valentina D’Alessandro, Christoph Starck, Volkmar Falk, Tomaso Zambelli, Julia A. Vorholt, Dimos Poulikakos, and Aldo Ferrari (2014). Toward a Rational Design of Surface Textures Promoting Endothelialization. Nano Lett. 14: 1069-1079; doi:10.1021/nl4047398
Eva Potthoff, Dario Ossola, Tomaso Zambelli, and Julia A. Vorholt (2015). Bacterial adhesion force quantification by fluidic force microscopy. Nanoscale 7: 4070; doi:10.1039/c4nr06495j
Leena Jaatinen, Eleanore Young, Jari Hyttinen, János Vörös, Tomaso Zambelli, and László Demkó (2016). Quantifying the effect of electric current on cell adhesion studied by single-cell force spectroscopy. Biointerphases 11: 011004; doi:10.1116/1.4940214
Noa Cohen, Saheli Sarkar, Evangelia Hondroulis, Pooja Sabhachandan, and Tania Konry (2017). Quantification of intercellular adhesion forces measured by fluid force microscopy. Talanta 174: 409-413; doi:10.1016/j.talanta.2017.06.038
Ana Sancho, Ine Vandersmissen, Sander Craps, Aernout Luttun, and Jürgen Groll (2017). A new strategy to measure intercellular adhesion forces in mature cell-cell contacts. Scientific Reports 7: 46152; doi:10.1038/srep46152

